State-dependent control of lumbar motoneurons by the hypocretinergic system
- PMID: 19962375
- PMCID: PMC2819009
- DOI: 10.1016/j.expneurol.2009.11.020
State-dependent control of lumbar motoneurons by the hypocretinergic system
Abstract
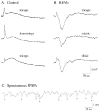
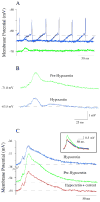
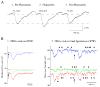
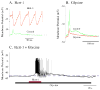
Neurons in the lateral hypothalamus (LH) that synthesize hypocretins (Hcrt-1 and Hcrt-2) are active during wakefulness and excite lumbar motoneurons. Because hypocretinergic cells also discharge during phasic periods of rapid eye movement (REM) sleep, we sought to examine their action on the activity of motoneurons during this state. Accordingly, cat lumbar motoneurons were intracellularly recorded, under alpha-chloralose anesthesia, prior to (control) and during the carbachol-induced REM sleep-like atonia (REMc). During control conditions, LH stimulation induced excitatory postsynaptic potentials (composite EPSP) in motoneurons. In contrast, during REMc, identical LH stimulation induced inhibitory PSPs in motoneurons. We then tested the effects of LH stimulation on motoneuron responses following the stimulation of the nucleus reticularis gigantocellularis (NRGc) which is part of a brainstem-spinal cord system that controls motoneuron excitability in a state-dependent manner. LH stimulation facilitated NRGc stimulation-induced composite EPSP during control conditions whereas it enhanced NRGc stimulation-induced IPSPs during REMc. These intriguing data indicate that the LH exerts a state-dependent control of motor activity. As a first step to understand these results, we examined whether hypocretinergic synaptic mechanisms in the spinal cord were state dependent. We found that the juxtacellular application of Hcrt-1 induced motoneuron excitation during control conditions whereas motoneuron inhibition was enhanced during REMc. These data indicate that the hypocretinergic system acts on motoneurons in a state-dependent manner via spinal synaptic mechanisms. Thus, deficits in Hcrt-1 may cause the coexistence of incongruous motor signs in cataplectic patients, such as motor suppression during wakefulness and movement disorders during REM sleep.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Effects of excitation of sensory pathways on the membrane potential of cat masseter motoneurons before and during cholinergically induced motor atonia.Neuroscience. 1998 Sep;86(2):557-69. doi: 10.1016/s0306-4522(98)00016-5. Neuroscience. 1998. PMID: 9881869
-
Hypocretinergic control of spinal cord motoneurons.J Neurosci. 2004 Jun 9;24(23):5336-45. doi: 10.1523/JNEUROSCI.4812-03.2004. J Neurosci. 2004. PMID: 15190106 Free PMC article.
-
Population synaptic potentials evoked in lumbar motoneurons following stimulation of the nucleus reticularis gigantocellularis during carbachol-induced atonia.Brain Res. 1994 Mar 14;639(2):313-9. doi: 10.1016/0006-8993(94)91745-0. Brain Res. 1994. PMID: 8205484
-
Synaptic mechanisms and circuitry involved in motoneuron control during sleep.Int Rev Neurobiol. 1983;24:213-58. doi: 10.1016/s0074-7742(08)60223-8. Int Rev Neurobiol. 1983. PMID: 6197386 Review.
-
The atonia and myoclonia of active (REM) sleep.Annu Rev Psychol. 1990;41:557-84. doi: 10.1146/annurev.ps.41.020190.003013. Annu Rev Psychol. 1990. PMID: 1968326 Review.
Cited by
-
The injection of hypocretin-1 into the nucleus pontis oralis induces either active sleep or wakefulness depending on the behavioral state when it is administered.Sleep. 2010 Sep;33(9):1236-43. doi: 10.1093/sleep/33.9.1236. Sleep. 2010. PMID: 20857871 Free PMC article.
-
Characterization of REM sleep without atonia in patients with narcolepsy and idiopathic hypersomnia using AASM scoring manual criteria.J Clin Sleep Med. 2013 Jul 15;9(7):675-80. doi: 10.5664/jcsm.2836. J Clin Sleep Med. 2013. PMID: 23853561 Free PMC article.
-
[Orexin-A promotes motor function recovery of rats with spinal cord injury by regulating ionotropic glutamate receptors].Nan Fang Yi Ke Da Xue Xue Bao. 2025 May 20;45(5):1023-1030. doi: 10.12122/j.issn.1673-4254.2025.05.15. Nan Fang Yi Ke Da Xue Xue Bao. 2025. PMID: 40415434 Free PMC article. Chinese.
References
-
- Amatruda TT, III, Black DA, McKenna TM, McCarley RW, Hobson JA. Sleep cycle control and cholinergic mechanisms: differential effects of carbachol injections at pontine brain stem sites. Brain Res. 1975;98:501–515. - PubMed
-
- Anand BK, Chhina GS, Singh B. Effect of glucose on the activity of hypothalamic “feeding centers”. Science. 1962;138:597–598. - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306:39–52. - PubMed
-
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA. A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res. 1987;414:245–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

