Phosphorylation and function of cardiac myosin binding protein-C in health and disease
- PMID: 19962384
- PMCID: PMC6800196
- DOI: 10.1016/j.yjmcc.2009.11.014
Phosphorylation and function of cardiac myosin binding protein-C in health and disease
Abstract
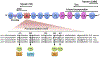
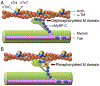
During the past 5 years there has been an increasing body of literature describing the roles cardiac myosin binding protein C (cMyBP-C) phosphorylation play in regulating cardiac function and heart failure. cMyBP-C is a sarcomeric thick filament protein that interacts with titin, myosin and actin to regulate sarcomeric assembly, structure and function. Elucidating the function of cMyBP-C is clinically important because mutations in this protein have been linked to cardiomyopathy in more than sixty million people worldwide. One function of cMyBP-C is to regulate cross-bridge formation through dynamic phosphorylation by protein kinase A, protein kinase C and Ca(2+)-calmodulin-activated kinase II, suggesting that cMyBP-C phosphorylation serves as a highly coordinated point of contractile regulation. Moreover, dephosphorylation of cMyBP-C, which accelerates its degradation, has been shown to associate with the development of heart failure in mouse models and in humans. Strikingly, cMyBP-C phosphorylation presents a potential target for therapeutic development as protection against ischemic-reperfusion injury, which has been demonstrated in mouse hearts. Also, emerging evidence suggests that cMyBP-C has the potential to be used as a biomarker for diagnosing myocardial infarction. Although many aspects of cMyBP-C phosphorylation and function remain poorly understood, cMyBP-C and its phosphorylation states have significant promise as a target for therapy and for providing a better understanding of the mechanics of heart function during health and disease. In this review we discuss the most recent findings with respect to cMyBP-C phosphorylation and function and determine potential future directions to better understand the functional role of cMyBP-C and phosphorylation in sarcomeric structure, myocardial contractility and cardioprotection.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure statement
There are no unlabeled/unapproved uses of drugs or products, and no real or apparent conflicts of interest to report.
Figures


References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009;119:480–6. - PubMed
-
- Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol 2007;42:247–59. - PubMed
-
- Jacques AM, Copeland O, Messer AE, Gallon CE, King K, McKenna WJ, et al. Myosin binding protein-C phosphorylation in normal, hypertrophic and failing human heart muscle. J Mol Cell Cardiol 2008;45:209–16. - PubMed
-
- Hamdani N, Paulus WJ, van Heerebeek L, Borbely A, Boontje NM, Zuidwijk MJ, et al. Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J 2009;30: 1863–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

