Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine
- PMID: 19962451
- PMCID: PMC2879579
- DOI: 10.1016/j.vph.2009.11.010
Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine
Abstract
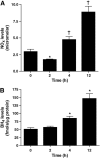
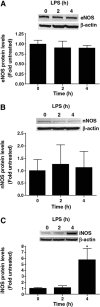
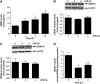
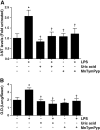
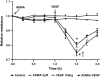
Acute lung injury (ALI) is associated with severe alterations in lung structure and function and is characterized by hypoxemia, pulmonary edema, low lung compliance and widespread capillary leakage. Asymmetric dimethylarginine (ADMA), a known cardiovascular risk factor, has been linked to endothelial dysfunction and the pathogenesis of a number of cardiovascular diseases. However, the role of ADMA in the pathogenesis of ALI is less clear. ADMA is metabolized via hydrolytic degradation to l-citrulline and dimethylamine by the enzyme, dimethylarginine dimethylaminohydrolase (DDAH). Recent studies suggest that lipopolysaccharide (LPS) markedly increases the level of ADMA and decreases DDAH activity in endothelial cells. Thus, the purpose of this study was to determine if alterations in the ADMA/DDAH pathway contribute to the development of ALI initiated by LPS-exposure in mice. Our data demonstrate that LPS exposure significantly increases ADMA levels and this correlates with a decrease in DDAH activity but not protein levels of either DDAH I or DDAH II isoforms. Further, we found that the increase in ADMA levels cause an early decrease in nitric oxide (NO(x)) and a significant increase in both NO synthase (NOS)-derived superoxide and total nitrated lung proteins. Finally, we found that decreasing peroxynitrite levels with either uric acid or Manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTymPyp) significantly attenuated the lung leak associated with LPS-exposure in mice suggesting a key role for protein nitration in the progression of ALI. In conclusion, this is the first study that suggests a role of the ADMA/DDAH pathway during the development of ALI in mice and that ADMA may be a novel therapeutic biomarker to ascertain the risk for development of ALI.
Figures
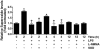

Similar articles
-
Hyper-activation of pp60Src limits nitric oxide signaling by increasing asymmetric dimethylarginine levels during acute lung injury.Free Radic Biol Med. 2017 Jan;102:217-228. doi: 10.1016/j.freeradbiomed.2016.11.008. Epub 2016 Nov 9. Free Radic Biol Med. 2017. PMID: 27838434 Free PMC article.
-
Dimethylarginine dimethylaminohydrolase II overexpression attenuates LPS-mediated lung leak in acute lung injury.Am J Respir Cell Mol Biol. 2014 Mar;50(3):614-25. doi: 10.1165/rcmb.2013-0193OC. Am J Respir Cell Mol Biol. 2014. PMID: 24134589 Free PMC article.
-
Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine-induced endothelial dysfunction in the cerebral circulation.Stroke. 2008 Jan;39(1):180-4. doi: 10.1161/STROKEAHA.107.490631. Epub 2007 Dec 6. Stroke. 2008. PMID: 18063827
-
Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems.Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3227-45. doi: 10.1152/ajpheart.00998.2007. Epub 2007 Oct 12. Am J Physiol Heart Circ Physiol. 2007. PMID: 17933965 Review.
-
The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis.Nat Rev Drug Discov. 2011 Apr;10(4):277-91. doi: 10.1038/nrd3358. Nat Rev Drug Discov. 2011. PMID: 21455237 Review.
Cited by
-
Perioperative "remote" acute lung injury: recent update.J Biomed Res. 2017 Jan 19;31(3):197-212. doi: 10.7555/JBR.31.20160053. J Biomed Res. 2017. PMID: 28808222 Free PMC article.
-
Reactive oxygen species in inflammation and tissue injury.Antioxid Redox Signal. 2014 Mar 1;20(7):1126-67. doi: 10.1089/ars.2012.5149. Epub 2013 Oct 22. Antioxid Redox Signal. 2014. PMID: 23991888 Free PMC article. Review.
-
Multiorgan Development of Oxidative and Nitrosative Stress in LPS-Induced Endotoxemia in C57Bl/6 Mice: DHE-Based In Vivo Approach.Oxid Med Cell Longev. 2019 May 22;2019:7838406. doi: 10.1155/2019/7838406. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31249650 Free PMC article.
-
Hyper-activation of pp60Src limits nitric oxide signaling by increasing asymmetric dimethylarginine levels during acute lung injury.Free Radic Biol Med. 2017 Jan;102:217-228. doi: 10.1016/j.freeradbiomed.2016.11.008. Epub 2016 Nov 9. Free Radic Biol Med. 2017. PMID: 27838434 Free PMC article.
-
Dimethylarginine dimethylaminohydrolase (DDAH) overexpression attenuates agricultural organic dust extract-induced inflammation.J Environ Immunol Toxicol. 2014 Mar;2(2):72-78. doi: 10.7178/jeit.15. J Environ Immunol Toxicol. 2014. PMID: 25221746 Free PMC article.
References
-
- Antoniades C, Shirodaria C, Leeson P, Antonopoulos A, Warrick N, Van-Assche T, Cunnington C, Tousoulis D, Pillai R, Ratnatunga C, Stefanadis C, Channon KM. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: implications for endothelial function in human atherosclerosis. Eur. Heart J. 2009;30:1142–1150. - PubMed
-
- Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of asymmetric dimethylarginines is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation. 2003;107:1195–1201. - PubMed
-
- Bae SW, Stuhlinger MC, Yoo HS, Yu KH, Park HK, Choi BY, Lee YS, Pachinger O, Choi YH, Lee SH, Park JE. Plasma asymmetric dimethylarginine concentrations in newly diagnosed patients with acute myocardial infarction or unstable angina pectoris during two weeks of medical treatment. Am. J. Cardiol. 2005;95:729–733. - PubMed
-
- Beckman JS. Protein tyrosine nitration and peroxynitrite. Faseb. J. 2002;16:1144. - PubMed
-
- Bevers LM, Braam B, Post JA, van Zonneveld AJ, Rabelink TJ, Koomans HA, Verhaar MC, Joles JA. Tetrahydrobiopterin, but not L-arginine, decreases NO synthase uncoupling in cells expressing high levels of endothelial NO synthase. Hypertension. 2006;47:87–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

