Tissue-penetrating delivery of compounds and nanoparticles into tumors
- PMID: 19962669
- PMCID: PMC2791543
- DOI: 10.1016/j.ccr.2009.10.013
Tissue-penetrating delivery of compounds and nanoparticles into tumors
Abstract
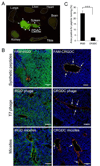
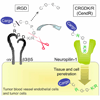
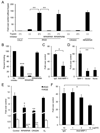
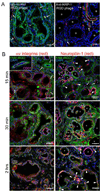
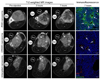
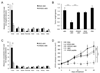
Poor penetration of drugs into tumors is a major obstacle in tumor treatment. We describe a strategy for peptide-mediated delivery of compounds deep into the tumor parenchyma that uses a tumor-homing peptide, iRGD (CRGDK/RGPD/EC). Intravenously injected compounds coupled to iRGD bound to tumor vessels and spread into the extravascular tumor parenchyma, whereas conventional RGD peptides only delivered the cargo to the blood vessels. iRGD homes to tumors through a three-step process: the RGD motif mediates binding to alphav integrins on tumor endothelium and a proteolytic cleavage then exposes a binding motif for neuropilin-1, which mediates penetration into tissue and cells. Conjugation to iRGD significantly improved the sensitivity of tumor-imaging agents and enhanced the activity of an antitumor drug.
Figures


References
-
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. - PubMed
-
- Arleth L, Ashok B, Onyuksel H, Thiyagarajan P, Jacob J, Hjelm RP. Detailed structure of hairy mixed micelles formed by phosphatidylcholine and PEGylated phospholipids in aqueous media. Langmuir. 2005;21:3279–3290. - PubMed
-
- Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 1987;262:17703–17711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

