Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge
- PMID: 19962744
- PMCID: PMC2850969
- DOI: 10.1016/j.jaci.2009.09.012
Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge
Abstract
Background: Omalizumab treatment suppresses FcepsilonRI expression faster on blood basophils than skin mast cells.
Objective: We used omalizumab to elucidate the relative contributions of basophil versus mast cell FcepsilonRI activation in a nasal allergen challenge (NAC) model.
Methods: Eighteen subjects with cat allergy were enrolled in a 3.5-month, double-blind, randomized (3.5:1), placebo-controlled trial of omalizumab using standard dosing. At baseline, subjects underwent NAC with lavage for prostaglandin D(2) measurement, skin prick test titration (SPTT), and blood sampling for basophil histamine release (BHR) and basophil IgE/FcepsilonRI measurements. Basophil studies were repeated at day 3 and then weekly until cat allergen-induced BHR was <20% of baseline or until day 45. Baseline visit procedures were repeated after the BHR reduction (midstudy NAC) and at the treatment period's completion (final NAC).
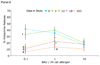
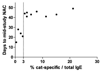
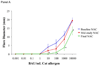
Results: Subjects treated with omalizumab who completed all NACs (n = 12) demonstrated significant mean reduction in BHR to an optimal dose of cat allergen by midstudy NAC compared with baseline (74% decrease; P = .001). In addition, these subjects demonstrated significant decreases in mean combined nasal symptom scores (50% decrease; P = .007) and total sneeze counts (59% decrease; P = .01) by midstudy NAC relative to baseline NAC. In contrast, measures of mast cell response (SPTT and nasal lavage prostaglandin D(2)) were only significantly reduced by the final NAC. Subjects on placebo (n = 4) did not experience a shift in basophil, NAC symptom, or mast cell measures.
Conclusion: Reduction in nasal symptom scores occurred when the basophil, but not mast cell, response was reduced on omalizumab, implicating a role for basophils in the acute NAC response.
Copyright (c) 2010 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures







References
-
- MacGlashan DW., Jr Therapeutic efficacy of omalizumab. J Allergy Clin Immunol. 2009;123:114–115. - PubMed
-
- Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354:2689–2695. - PubMed
-
- Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, et al. Humanization of an antibody directed against IgE. J Immunol. 1993;151:2623–2632. - PubMed
-
- Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med. 2001;164:S6–S11. - PubMed
-
- MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–1445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

