Functional diversity of ankyrin repeats in microbial proteins
- PMID: 19962898
- PMCID: PMC2834824
- DOI: 10.1016/j.tim.2009.11.004
Functional diversity of ankyrin repeats in microbial proteins
Abstract
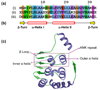
The ankyrin repeat (ANK) is the most common protein-protein interaction motif in nature, and is predominantly found in eukaryotic proteins. Genome sequencing of various pathogenic or symbiotic bacteria and eukaryotic viruses has identified numerous genes encoding ANK-containing proteins that are proposed to have been acquired from eukaryotes by horizontal gene transfer. However, the recent discovery of additional ANK-containing proteins encoded in the genomes of archaea and free-living bacteria suggests either a more ancient origin of the ANK motif or multiple convergent evolution events. Many bacterial pathogens employ various types of secretion systems to deliver ANK-containing proteins into eukaryotic cells, where they mimic or manipulate various host functions. Studying the molecular and biochemical functions of this family of proteins will enhance our understanding of important host-microbe interactions.
Figures
Similar articles
-
Chlorovirus Skp1-binding ankyrin repeat protein interplay and mimicry of cellular ubiquitin ligase machinery.J Virol. 2014 Dec;88(23):13798-810. doi: 10.1128/JVI.02109-14. Epub 2014 Sep 24. J Virol. 2014. PMID: 25253343 Free PMC article.
-
Poxviral ankyrin proteins.Viruses. 2015 Feb 16;7(2):709-38. doi: 10.3390/v7020709. Viruses. 2015. PMID: 25690795 Free PMC article. Review.
-
Orientia tsutsugamushi Strain Ikeda Ankyrin Repeat-Containing Proteins Recruit SCF1 Ubiquitin Ligase Machinery via Poxvirus-Like F-Box Motifs.J Bacteriol. 2015 Oct;197(19):3097-109. doi: 10.1128/JB.00276-15. Epub 2015 Jul 13. J Bacteriol. 2015. PMID: 26170417 Free PMC article.
-
Ankyrin domains across the Tree of Life.PeerJ. 2014 Feb 6;2:e264. doi: 10.7717/peerj.264. eCollection 2014. PeerJ. 2014. PMID: 24688847 Free PMC article.
-
[Homologous protein domains in superkingdoms Archaea, Bacteria, and Eukaryota and the problem of the origin of eukaryotes].Izv Akad Nauk Ser Biol. 2005 Jul-Aug;(4):389-400. Izv Akad Nauk Ser Biol. 2005. PMID: 16212260 Review. Russian.
Cited by
-
A bacterial protein promotes the recognition of the Legionella pneumophila vacuole by autophagy.Eur J Immunol. 2013 May;43(5):1333-44. doi: 10.1002/eji.201242835. Epub 2013 Apr 8. Eur J Immunol. 2013. PMID: 23420491 Free PMC article.
-
Nothing in Evolution Makes Sense Except in the Light of Genomics: Read-Write Genome Evolution as an Active Biological Process.Biology (Basel). 2016 Jun 8;5(2):27. doi: 10.3390/biology5020027. Biology (Basel). 2016. PMID: 27338490 Free PMC article. Review.
-
Molecular Pathotyping of Plasmodiophora brassicae-Genomes, Marker Genes, and Obstacles.Pathogens. 2021 Feb 24;10(3):259. doi: 10.3390/pathogens10030259. Pathogens. 2021. PMID: 33668372 Free PMC article. Review.
-
Identification of a new rhoptry neck complex RON9/RON10 in the Apicomplexa parasite Toxoplasma gondii.PLoS One. 2012;7(3):e32457. doi: 10.1371/journal.pone.0032457. Epub 2012 Mar 12. PLoS One. 2012. PMID: 22427839 Free PMC article.
-
The diversity and evolution of Wolbachia ankyrin repeat domain genes.PLoS One. 2013;8(2):e55390. doi: 10.1371/journal.pone.0055390. Epub 2013 Feb 4. PLoS One. 2013. PMID: 23390535 Free PMC article.
References
-
- Bork P. Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Proteins. 1993;17(4):363–374. - PubMed
-
- Kitazawa M, et al. Intracellular cAMP controls a physical association of V-1 with CapZ in cultured mammalian endocrine cells. Biochem Biophys Res Commun. 2005;331(1):181–186. - PubMed
-
- Foord R, et al. X-ray structural analysis of the yeast cell cycle regulator Swi6 reveals variations of the ankyrin fold and has implications for Swi6 function. Nat Struct Biol. 1999;6(2):157–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

