The effect of spermine on the initiation of mitochondrial protein synthesis
- PMID: 19962967
- PMCID: PMC2812669
- DOI: 10.1016/j.bbrc.2009.11.169
The effect of spermine on the initiation of mitochondrial protein synthesis
Abstract
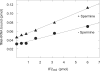
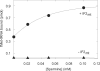
Polyamines are important in both prokaryotic and eukaryotic translational systems. Spermine is a quaternary aliphatic amine that is cationic under physiological conditions. In this paper, we demonstrate that spermine stimulates fMet-tRNA binding to mammalian mitochondrial 55S ribosomes. The stimulatory effect of spermine is independent of the identity of the mRNA. The degree of stimulation of spermine is the same at all concentrations of mitochondrial initiation factor 2 (IF2(mt)) and mitochondrial initiation factor 3 (IF3(mt)). This observation indicates that IF2(mt) and IF3(mt), while essential for initiation, are not the primary components of the translation initiation system affected by spermine. IF3(mt) dissociates 55S ribosomes detectably in the absence of spermine, but this effect is strongly inhibited in the presence of spermine. This observation indicates that the positive effect of spermine on initiation is not due to an increase in the availability of the small subunits for initiation. Spermine also promotes fMet-tRNA binding to small subunits of the mitochondrial ribosome in the presence of IF2(mt). The major effect of spermine in promoting initiation complex formation thus appears to be on the interaction of fMet-tRNA with the ribosome.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

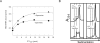
References
-
- Spremulli LL, Coursey A, Navratil T, Hunter SE. Initiation and elongation factors in mammalian mitochondrial protein synthesis. In: Moldave K, editor. Prog. Nuc. Acid Res. Mol. Biol. Elsevier; 2004. pp. 211–261. - PubMed
-
- Koc EC, Spremulli LL. Identification of mammalian mitochondrial translational initiation factor 3 and examination of its role in initiation complex formation with natural mRNAs. J. Biol. Chem. 2002;277:35541–35549. - PubMed
-
- Liao H-X, Spremulli LL. Initiation of protein synthesis in animal mitochondria: Purification and characterization of translational initiation factor 2. J. Biol. Chem. 1991;266:20714–20719. - PubMed
-
- Takeda Y, Samejima K, Nagano K, Watanabe M, Sugeta H, Kyogoku Y. Determination of protonation sites in thermospermine and in some other polyamines by 15N and 13C nuclear magnetic resonance spectroscopy. Eur. J. Biochem. 1983;130:383–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

