DeNAno: Selectable deoxyribonucleic acid nanoparticle libraries
- PMID: 19963022
- PMCID: PMC2822443
- DOI: 10.1016/j.jbiotec.2009.12.002
DeNAno: Selectable deoxyribonucleic acid nanoparticle libraries
Abstract
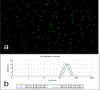
DNA nanoparticles of approximately 250 nm were produced by rolling circle replication of circular oligonucleotide templates which results in highly condensed DNA particulates presenting concatemeric sequence repeats. Using templates containing randomized sequences, high diversity libraries of particles were produced. A biopanning method that iteratively screens for binding and uses PCR to recover selected particles was developed. The initial application of this technique was the selection of particles that bound to human dendritic cells (DCs). Following nine rounds of selection the population of particles was enriched for particles that bound DCs, and individual binding clones were isolated and confirmed by flow cytometry and microscopy. This process, which we have termed DeNAno, represents a novel library technology akin to aptamer and phage display, but unique in that the selected moiety is a multivalent nanoparticle whose activity is intrinsic to its sequence. Cell targeted DNA nanoparticles may have applications in cell imaging, cell sorting, and cancer therapy.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures
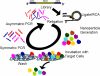


Similar articles
-
Selection of DNA nanoparticles with preferential binding to aggregated protein target.Nucleic Acids Res. 2016 Jun 2;44(10):e96. doi: 10.1093/nar/gkw136. Epub 2016 Mar 11. Nucleic Acids Res. 2016. PMID: 26969734 Free PMC article.
-
[Peptide phage display in biotechnology and biomedicine].Biomed Khim. 2016 Jul;62(5):481-495. doi: 10.18097/PBMC20166205481. Biomed Khim. 2016. PMID: 27797323 Review. Russian.
-
Phage-displayed combinatorial peptide libraries in fusion to beta-lactamase as reporter for an accelerated clone screening: Potential uses of selected enzyme-linked affinity reagents in downstream applications.Comb Chem High Throughput Screen. 2010 Jan;13(1):75-87. doi: 10.2174/138620710790218258. Comb Chem High Throughput Screen. 2010. PMID: 20214576 Free PMC article.
-
An improved SELEX technique for selection of DNA aptamers binding to M-type 11 of Streptococcus pyogenes.Methods. 2016 Mar 15;97:51-7. doi: 10.1016/j.ymeth.2015.12.005. Epub 2015 Dec 8. Methods. 2016. PMID: 26678795
-
Phage display: concept, innovations, applications and future.Biotechnol Adv. 2010 Nov-Dec;28(6):849-58. doi: 10.1016/j.biotechadv.2010.07.004. Epub 2010 Jul 23. Biotechnol Adv. 2010. PMID: 20659548 Review.
Cited by
-
Selection of DNA nanoparticles with preferential binding to aggregated protein target.Nucleic Acids Res. 2016 Jun 2;44(10):e96. doi: 10.1093/nar/gkw136. Epub 2016 Mar 11. Nucleic Acids Res. 2016. PMID: 26969734 Free PMC article.
-
Multivalent Aptamers: Versatile Tools for Diagnostic and Therapeutic Applications.Molecules. 2016 Nov 25;21(12):1613. doi: 10.3390/molecules21121613. Molecules. 2016. PMID: 27898020 Free PMC article. Review.
-
Development of DNA aptamers targeting B7H3 by hybrid-SELEX: an alternative to antibodies for immuno-assays.Sci Rep. 2024 Jun 12;14(1):13552. doi: 10.1038/s41598-024-64559-7. Sci Rep. 2024. PMID: 38866941 Free PMC article.
-
Facile Display of Homomultivalent Proteins for In Vitro Selections.ACS Synth Biol. 2023 Feb 17;12(2):634-638. doi: 10.1021/acssynbio.2c00563. Epub 2023 Jan 19. ACS Synth Biol. 2023. PMID: 36655840 Free PMC article.
References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. - PubMed
-
- Wilson DS, Szostak JW. In vitro selection of functional nucleic acids. Annu. Rev. Biochem. 1999;68:611–647. - PubMed
-
- Smith GP, Scott JK. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 1993;217:228–257. - PubMed
-
- Clackson T, Hoogenboom HR, Griffiths AD, Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

