Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds
- PMID: 19963268
- PMCID: PMC2813987
- DOI: 10.1016/j.biomaterials.2009.11.058
Dose effect of tumor necrosis factor-alpha on in vitro osteogenic differentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds
Abstract
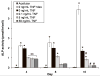
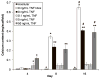
This study presents a first step in the development of a bone tissue engineering strategy to trigger enhanced osteogenesis by modulating inflammation. This work focused on characterizing the effects of the concentration of a pro-inflammatory cytokine, tumor necrosis factor alpha (TNF-alpha), on osteogenic differentiation of mesenchymal stem cells (MSCs) grown in a 3D culture system. MSC osteogenic differentiation is typically achieved in vitro through a combination of osteogenic supplements that include the anti-inflammatory corticosteroid dexamethasone. Although simple, the use of dexamethasone is not clinically realistic, and also hampers in vitro studies of the role of inflammatory mediators in wound healing. In this study, MSCs were pre-treated with dexamethasone to induce osteogenic differentiation, and then cultured in biodegradable electrospun poly(epsilon-caprolactone) (PCL) scaffolds, which supported continued MSC osteogenic differentiation in the absence of dexamethasone. Continuous delivery of 0.1 ng/mL of recombinant rat TNF-alpha suppressed osteogenic differentiation of rat MSCs over 16 days, which was likely the result of residual dexamethasone antagonizing TNF-alpha signaling. Continuous delivery of a higher dose, 5 ng/mL TNF-alpha, stimulated osteogenic differentiation for a few days, and 50 ng/mL TNF-alpha resulted in significant mineralized matrix deposition over the course of the study. These findings suggest that the pro-inflammatory cytokine TNF-alpha stimulates osteogenic differentiation of MSCs, an effect that can be blocked by the presence of anti-inflammatory agents like dexamethasone, with significant implications on the interplay between inflammation and tissue regeneration.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures

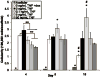


Similar articles
-
Effect of temporally patterned TNF-α delivery on in vitro osteogenic differentiation of mesenchymal stem cells cultured on biodegradable polymer scaffolds.J Biomater Sci Polym Ed. 2013;24(15):1794-813. doi: 10.1080/09205063.2013.803455. Epub 2013 Jun 8. J Biomater Sci Polym Ed. 2013. PMID: 23746285 Free PMC article.
-
Student Award for Outstanding Research Winner in the Ph.D. Category for the 9th World Biomaterials Congress, Chengdu, China, June 1-5, 2012: The interplay of bone-like extracellular matrix and TNF-α signaling on in vitro osteogenic differentiation of mesenchymal stem cells.J Biomed Mater Res A. 2012 May;100(5):1097-106. doi: 10.1002/jbm.a.34058. Epub 2012 Feb 18. J Biomed Mater Res A. 2012. PMID: 22345065 Free PMC article.
-
Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements.Tissue Eng Part A. 2010 Feb;16(2):431-40. doi: 10.1089/ten.TEA.2009.0583. Tissue Eng Part A. 2010. PMID: 19863274 Free PMC article.
-
Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors.PLoS One. 2011;6(10):e26211. doi: 10.1371/journal.pone.0026211. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022571 Free PMC article.
-
Nanoclay-enriched poly(ɛ-caprolactone) electrospun scaffolds for osteogenic differentiation of human mesenchymal stem cells.Tissue Eng Part A. 2014 Aug;20(15-16):2088-101. doi: 10.1089/ten.tea.2013.0281. Epub 2014 May 19. Tissue Eng Part A. 2014. PMID: 24842693 Free PMC article.
Cited by
-
Classical and Paradoxical Effects of TNF-α on Bone Homeostasis.Front Immunol. 2014 Feb 13;5:48. doi: 10.3389/fimmu.2014.00048. eCollection 2014. Front Immunol. 2014. PMID: 24592264 Free PMC article. Review.
-
NF-κB as a Therapeutic Target in Inflammatory-Associated Bone Diseases.Adv Protein Chem Struct Biol. 2017;107:117-154. doi: 10.1016/bs.apcsb.2016.11.002. Epub 2016 Dec 9. Adv Protein Chem Struct Biol. 2017. PMID: 28215222 Free PMC article. Review.
-
Inflammatory niche: Mesenchymal stromal cell priming by soluble mediators.World J Stem Cells. 2020 Sep 26;12(9):922-937. doi: 10.4252/wjsc.v12.i9.922. World J Stem Cells. 2020. PMID: 33033555 Free PMC article. Review.
-
ELP2 negatively regulates osteoblastic differentiation impaired by tumor necrosis factor α in MC3T3-E1 cells through STAT3 activation.J Cell Physiol. 2019 Aug;234(10):18075-18085. doi: 10.1002/jcp.28440. Epub 2019 Mar 7. J Cell Physiol. 2019. PMID: 30847950 Free PMC article.
-
Lidocaine intensifies the anti-osteogenic effect on inflammation-induced human dental pulp stem cells via mitogen-activated protein kinase inhibition.J Dent Sci. 2023 Jul;18(3):1062-1072. doi: 10.1016/j.jds.2022.11.020. Epub 2022 Dec 1. J Dent Sci. 2023. PMID: 37404644 Free PMC article.
References
-
- Ingber DE, Levin M. What lies at the interface of regenerative medicine and developmental biology. Development. 2007;134:2541–2547. - PubMed
-
- Rundle CH, Wang H, Yu H, Chadwick RB, Davis EI, Wegedal JE, et al. Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone. 2006;38:521–529. - PubMed
-
- Pham QP, Kasper FK, Baggett LS, Raphael RM, Jansen JA, Mikos AG. The influence of the in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29(18):2729–2739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

