Microtubule-severing enzymes
- PMID: 19963362
- PMCID: PMC2822099
- DOI: 10.1016/j.ceb.2009.11.001
Microtubule-severing enzymes
Abstract
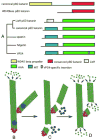
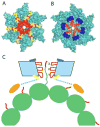
In 1993, an enzyme with an ATP-dependent microtubule-severing activity was purified from sea urchin eggs and named katanin, after the Japanese word for sword. Now we know that katanin, spastin, and fidgetin form a family of closely related microtubule-severing enzymes that is widely distributed in eukaryotes ranging from Tetrahymena and Chlamydomonas to humans. Here we review the diverse in vivo functions of these proteins and the recent significant advances in deciphering the biophysical mechanism of microtubule severing.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
References
-
- Hartman JJ, Mahr J, McNally K, Okawa K, Iwamatsu A, Thomas S, Cheesman S, Heuser J, Vale RD, McNally FJ. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell. 1998;93:277–287. - PubMed
-
- Roll-Mecak A, Vale RD. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr Biol. 2005;15:650–655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

