Lensless imaging for point-of-care testing
- PMID: 19964416
- PMCID: PMC3774534
- DOI: 10.1109/IEMBS.2009.5333765
Lensless imaging for point-of-care testing
Abstract
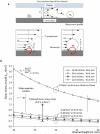
We show a platform that merges a microfluidic chip with lensless imaging for CD4(+) T-lymphocyte counting at resource-limited settings. To capture CD4(+) T lymphocytes, anti-CD4 antibody was immobilized on a microfluidic chip. The captured cells were detected by a charge coupled device (CCD) sensor using lensless shadow imaging techniques. Gray scale shadow images of captured cells on the chip (24 mm x 4 mm x 50 mum) were enumerated in three seconds using an automatic cell counting software. The device achieved 70.2 +/- 6.5% capture efficiency, 88.8 +/- 5.4% capture specificity for CD4(+) T-lymphocytes, 96 +/- 1.6% CCD efficiency, and 83.5 +/- 2.4% overall platform performance (n = 9 devices). This integrated platform has potential for point-of-care testing (POCT) to rapidly capture, image and count specific cell types from unprocessed whole blood.
Figures


References
-
- WHO . 2007 UNAIDS annual report: Knowing your epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS); GENEVA: 2008.
-
- Hammer SM, Eron JJ, Jr., Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JSG, Richman DD, Yeni PG, Volberding PA. Antiretroviral Treatment of Adult HIV Infection: 2008 Recommendations of the International AIDS Society-USA Panel. JAMA. 2008 Aug 6;300:555–570. 2008. - PubMed
-
- WHO . Patient Monitoring Guidelines for HIV Care and Antiretroviral Therapy. WHO; GENEVA: 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
