Monitoring intramyocardial reentry using alternating transillumination
- PMID: 19964628
- PMCID: PMC2895618
- DOI: 10.1109/IEMBS.2009.5334048
Monitoring intramyocardial reentry using alternating transillumination
Abstract
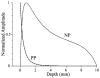
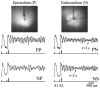
Intramyocardial reentry is implicated as a primary cause of the most deadly cardiac arrhythmias known as polymorphic ventricular tachycardia and ventricular fibrillation. However, the mechanisms involved in the triggering of such reentry and controlling its subsequent dynamics remain poorly understood. One of the major obstacles has been a lack of adequate tools that would enable 3D imaging of electrical excitation and reentry inside thick ventricular wall. Here, we present a new experimental technique, termed alternating transillumination (AT), aimed at filling this gap. The AT technique utilizes a recently synthesized near-infrared fluorescent voltage-sensitive dye, DI-4-ANBDQBS. We apply AT to study the dynamics of reentry during shock-induced polymorphic ventricular tachycardia in pig myocardium.
Figures


References
-
- Rosenbaum DS, Jalife J. Optical mapping of cardiac excitation and arrhythmias. Wiley; 2001.
-
- Khait VD, Bernus O, Mironov SF, Pertsov AM. Method for the three-dimensional localization of intramyocardial excitation centers using optical imaging. Journal of Biomedical Optics. 2006;11:034007. - PubMed
-
- DPZMD, JJMD . Cardiac Electrophysiology: From Cell to Bedside: Expert Consult - Online and Print. Saunders; 2009.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
