Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C-H olefination
- PMID: 19965380
- PMCID: PMC2879878
- DOI: 10.1126/science.1182512
Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C-H olefination
Abstract
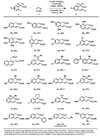
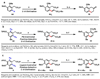
The Mizoroki-Heck reaction, which couples aryl halides with olefins, has been widely used to stitch together the carbogenic cores of numerous complex organic molecules. Given that the position-selective introduction of a halide onto an arene is not always straightforward, direct olefination of aryl carbon-hydrogen (C-H) bonds would obviate the inefficiencies associated with generating halide precursors or their equivalents. However, methods for carrying out such a reaction have suffered from narrow substrate scope and low positional selectivity. We report an operationally simple, atom-economical, carboxylate-directed Pd(II)-catalyzed C-H olefination reaction with phenylacetic acid and 3-phenylpropionic acid substrates, using oxygen at atmospheric pressure as the oxidant. The positional selectivity can be tuned by introducing amino acid derivatives as ligands. We demonstrate the versatility of the method through direct elaboration of commercial drug scaffolds and efficient syntheses of 2-tetralone and naphthoic acid natural product cores.
Figures


References
-
- Dick AR, Sanford MS. Tetrahedron. 2006;62:2439.
-
- Daugulis O, Zaitsev VG, Shabashov D, Pham Q-N, Lazareva A. Synlett. 2006:3382.
-
- Campeau L-C, Stuart DR, Fagnou K. Aldrichimica Acta. 2007;40:35.
-
- Yu J-Q, Giri R, Chen X. Org. Biomol. Chem. 2006;4:4041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

