Cell signaling in space and time: where proteins come together and when they're apart
- PMID: 19965465
- PMCID: PMC3041271
- DOI: 10.1126/science.1175668
Cell signaling in space and time: where proteins come together and when they're apart
Abstract
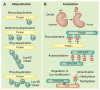
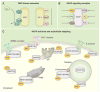
Signal transduction can be defined as the coordinated relay of messages derived from extracellular cues to intracellular effectors. More simply put, information received on the cell surface is processed across the plasma membrane and transmitted to intracellular targets. This requires that the activators, effectors, enzymes, and substrates that respond to cellular signals come together when they need to.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

