A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression
- PMID: 19965510
- PMCID: PMC3428834
- DOI: 10.1126/science.1175326
A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression
Abstract
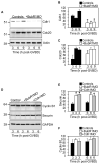
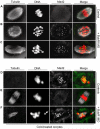
Two critical stages of mammalian oocyte regulation are prophase I arrest, which is important for sustaining the oocyte pool, and the progression through meiosis I (MI) to produce fertilizable eggs. We have found that the spindle assembly checkpoint protein BubR1 regulates both stages in mouse oocytes. We show that oocytes depleted of BubR1 cannot sustain prophase I arrest and readily undergo germinal vesicle breakdown, a marker for reentry into MI. BubR1-depleted oocytes then arrest before completing MI, marked by failure of polar body extrusion. Both meiotic defects in BubR1-depleted oocytes are due to reduced activity of the master regulator known as the anaphase-promoting complex (APC), brought about through diminished levels of the APC coactivator Cdh1.
Figures


Similar articles
-
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells.Mol Hum Reprod. 2010 Sep;16(9):654-64. doi: 10.1093/molehr/gaq034. Epub 2010 May 7. Mol Hum Reprod. 2010. PMID: 20453035 Free PMC article. Review.
-
Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes.Nat Cell Biol. 2007 Oct;9(10):1192-8. doi: 10.1038/ncb1640. Epub 2007 Sep 23. Nat Cell Biol. 2007. PMID: 17891138 Free PMC article.
-
Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes.Development. 2012 Jun;139(11):1941-6. doi: 10.1242/dev.078352. Epub 2012 Apr 18. Development. 2012. PMID: 22513372 Free PMC article.
-
Hec1-dependent cyclin B2 stabilization regulates the G2-M transition and early prometaphase in mouse oocytes.Dev Cell. 2013 Apr 15;25(1):43-54. doi: 10.1016/j.devcel.2013.02.008. Epub 2013 Mar 28. Dev Cell. 2013. PMID: 23541922 Free PMC article.
-
Cyclin A and Nek2A: APC/C-Cdc20 substrates invisible to the mitotic spindle checkpoint.Biochem Soc Trans. 2010 Feb;38(Pt 1):72-7. doi: 10.1042/BST0380072. Biochem Soc Trans. 2010. PMID: 20074038 Review.
Cited by
-
polo Is Identified as a Suppressor of bubR1 Nondisjunction in a Deficiency Screen of the Third Chromosome in Drosophila melanogaster.G3 (Bethesda). 2011 Jul;1(2):161-9. doi: 10.1534/g3.111.000265. Epub 2011 Jul 1. G3 (Bethesda). 2011. PMID: 22384328 Free PMC article.
-
APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing.Mol Biol Cell. 2012 Oct;23(20):3970-81. doi: 10.1091/mbc.E12-05-0352. Epub 2012 Aug 23. Mol Biol Cell. 2012. PMID: 22918942 Free PMC article.
-
Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells.Mol Hum Reprod. 2010 Sep;16(9):654-64. doi: 10.1093/molehr/gaq034. Epub 2010 May 7. Mol Hum Reprod. 2010. PMID: 20453035 Free PMC article. Review.
-
A PP2A-B56-Centered View on Metaphase-to-Anaphase Transition in Mouse Oocyte Meiosis I.Cells. 2020 Feb 7;9(2):390. doi: 10.3390/cells9020390. Cells. 2020. PMID: 32046180 Free PMC article. Review.
-
Dual-mode regulation of the APC/C by CDK1 and MAPK controls meiosis I progression and fidelity.J Cell Biol. 2014 Mar 17;204(6):891-900. doi: 10.1083/jcb.201305049. J Cell Biol. 2014. PMID: 24637322 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

