Effects of human follicular fluid and high-density lipoproteins on early spermatozoa hyperactivation and cholesterol efflux
- PMID: 19965575
- PMCID: PMC3035499
- DOI: 10.1194/jlr.M000679
Effects of human follicular fluid and high-density lipoproteins on early spermatozoa hyperactivation and cholesterol efflux
Abstract
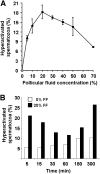
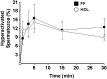
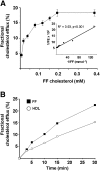
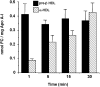
The preovulatory human follicular fluid contains only HDLs as a lipoprotein class with a typically high proportion of prebeta HDL. We first examined the role of follicular fluid and HDL subfractions on human spermatozoa capacitation, a process characterized by a hyperactivation of the flagellar movement and a depletion of plasma membrane cholesterol. Whole follicular fluid and isolated HDL, used at constant free cholesterol concentration, were both able to promote an early flagellar hyperactivation. Moreover, incubation of [(3)H]cholesterol-labeled spermatozoa with follicular fluid induced a rapid cholesterol efflux from spermatozoa that was confirmed by mass measurements of cholesterol transfer. Using isolated HDL, the cholesterol efflux had a similar time course and represented 70% of that mediated by whole follicular fluid. We then analyzed the time course of radioactive labeling of HDL subfractions. In the first minute of incubation, we found that the prebeta HDL fraction incorporated the main part of the radioactivity (60%), with the rest being found in alpha-HDL, but strikingly, the labeling of alpha-HDL increased with time at the expense of prebeta HDL.Thus, our results indicate that HDLs are involved in both spermatozoa hyperactivation and cholesterol effl ux and suggest the role of prebeta-HDL particles as fi rst cellular cholesterol acceptors.
Figures
Similar articles
-
Structural and functional comparison of HDL from homologous human plasma and follicular fluid. A model for extravascular fluid.Arterioscler Thromb Vasc Biol. 1997 Aug;17(8):1605-13. doi: 10.1161/01.atv.17.8.1605. Arterioscler Thromb Vasc Biol. 1997. PMID: 9301642
-
Identification of sterol acceptors that stimulate cholesterol efflux from human spermatozoa during in vitro capacitation.Gamete Res. 1988 Jun;20(2):185-201. doi: 10.1002/mrd.1120200209. Gamete Res. 1988. PMID: 3235036
-
High-Density Lipoprotein Particles, Cell-Cholesterol Efflux, and Coronary Heart Disease Risk.Arterioscler Thromb Vasc Biol. 2018 Sep;38(9):2007-2015. doi: 10.1161/ATVBAHA.118.311117. Arterioscler Thromb Vasc Biol. 2018. PMID: 30002062 Free PMC article.
-
Cholesterol efflux from macrophages and other cells.Curr Opin Lipidol. 1996 Oct;7(5):308-19. doi: 10.1097/00041433-199610000-00009. Curr Opin Lipidol. 1996. PMID: 8937522 Review.
-
Prebeta-1 HDL and coronary heart disease.Curr Opin Lipidol. 2012 Aug;23(4):367-71. doi: 10.1097/MOL.0b013e328353eef1. Curr Opin Lipidol. 2012. PMID: 22517613 Review.
Cited by
-
HDL mediates reverse cholesterol transport from ram spermatozoa and induces hyperactivated motility.Biol Reprod. 2021 Jun 4;104(6):1271-1281. doi: 10.1093/biolre/ioab035. Biol Reprod. 2021. PMID: 33674849 Free PMC article.
-
BODIPY-cholesterol can be reliably used to monitor cholesterol efflux from capacitating mammalian spermatozoa.Sci Rep. 2019 Jul 8;9(1):9804. doi: 10.1038/s41598-019-45831-7. Sci Rep. 2019. PMID: 31285440 Free PMC article.
-
Male Infertility: Shining a Light on Lipids and Lipid-Modulating Enzymes in the Male Germline.J Clin Med. 2020 Jan 23;9(2):327. doi: 10.3390/jcm9020327. J Clin Med. 2020. PMID: 31979378 Free PMC article. Review.
-
Epigenetics Role in Spermatozoa Function: Implications in Health and Evolution-An Overview.Life (Basel). 2023 Jan 29;13(2):364. doi: 10.3390/life13020364. Life (Basel). 2023. PMID: 36836724 Free PMC article. Review.
References
-
- Hoshi K., Aita T., Yanagida K., Yoshimatsu N., Sato A. 1990. Variation in the cholesterol/phospholipid ratio in human spermatozoa and its relationship with capacitation. Hum. Reprod. 5: 71–74. - PubMed
-
- Benoff S. 1993. Preliminaries to fertilization. The role of cholesterol during capacitation of human spermatozoa. Hum. Reprod. 8: 2001–2006. - PubMed
-
- Cross N. L. 1998. Role of cholesterol in sperm capacitation. Biol. Reprod. 59: 7–11. - PubMed
-
- Gamzu R., Yogev L., Paz G., Yavetz H., Lichtenberg D. 1997. Reduction of sperm cholesterol:phospholipid ratio is a possible mechanism for enhancement of human sperm binding to the zona pellucida following incubation with phosphatidylcholine liposomes. Biol. Reprod. 57: 539–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

