Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450
- PMID: 19965576
- PMCID: PMC2853439
- DOI: 10.1194/jlr.M003061
Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450
Abstract
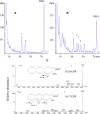
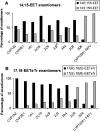
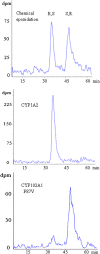
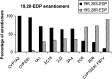
Cytochromes P450 (CYPs) metabolize polyunsaturated long-chain fatty acids (PUFA-LC) to several classes of oxygenated metabolites. Through use of human recombinant CYPs, we recently showed that CYP1A1, -2C19, -2D6, -2E1, and -3A4 are mainly hydroxylases, whereas CYP1A2, -2C8, -2C9, and -2J2 are mainly epoxygenases of arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), respectively. It is worth noting that the last double bond of these PUFAs, i.e., omega6 in AA or omega3 in EPA and DHA, respectively, was preferentially epoxidized. In this study, we have characterized the stereoselectivity of this epoxidation reaction by comparison with the PUFA-LC epoxide stereoisomers obtained from the enantioselective bacterial CYP102A1 F87V. The stereoselectivity of the epoxidation of the last olefin of AA (omega6), EPA (omega3), or DHA (omega3) differed between the CYP isoforms but was similar for EPA and DHA. These data give additional insight into the PUFA-LC epoxide enantiomers generated by the hepatic CYPs.
Figures
Similar articles
-
Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450.Arch Biochem Biophys. 2008 Mar 15;471(2):116-25. doi: 10.1016/j.abb.2008.01.002. Epub 2008 Jan 11. Arch Biochem Biophys. 2008. PMID: 18206980
-
Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids.Biochim Biophys Acta. 2011 Jan;1814(1):210-22. doi: 10.1016/j.bbapap.2010.09.009. Epub 2010 Sep 30. Biochim Biophys Acta. 2011. PMID: 20869469
-
Enzymatic Synthesis of Epoxidized Metabolites of Docosahexaenoic, Eicosapentaenoic, and Arachidonic Acids.J Vis Exp. 2019 Jun 28;(148):10.3791/59770. doi: 10.3791/59770. J Vis Exp. 2019. PMID: 31305515 Free PMC article.
-
Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids.Pharmacol Rep. 2010 May-Jun;62(3):536-47. doi: 10.1016/s1734-1140(10)70311-x. Pharmacol Rep. 2010. PMID: 20631419 Review.
-
Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism.Biochim Biophys Acta. 2015 Apr;1851(4):356-65. doi: 10.1016/j.bbalip.2014.07.020. Epub 2014 Aug 2. Biochim Biophys Acta. 2015. PMID: 25093613 Free PMC article. Review.
Cited by
-
Distinctive Metabolism-Associated Gene Clusters That Are Also Prognostic in Intrahepatic Cholangiocarcinoma and Hepatocellular Carcinoma.Oxid Med Cell Longev. 2022 Sep 19;2022:6595989. doi: 10.1155/2022/6595989. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2023 Aug 2;2023:9815947. doi: 10.1155/2023/9815947. PMID: 36199423 Free PMC article. Retracted.
-
Impact of e-cigarette aerosol on primary human alveolar epithelial type 2 cells.Am J Physiol Lung Cell Mol Physiol. 2022 Aug 1;323(2):L152-L164. doi: 10.1152/ajplung.00503.2021. Epub 2022 Jun 7. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 35670478 Free PMC article.
-
Periodontal inflammation potentially inhibits hepatic cytochrome P450 expression and disrupts the omega-3 epoxidation pathway in a murine model.J Dent Sci. 2025 Jan;20(1):444-451. doi: 10.1016/j.jds.2024.05.028. Epub 2024 May 29. J Dent Sci. 2025. PMID: 39873042 Free PMC article. No abstract available.
-
Pharmacogenetics and Forensic Toxicology: A New Step towards a Multidisciplinary Approach.Toxics. 2021 Nov 4;9(11):292. doi: 10.3390/toxics9110292. Toxics. 2021. PMID: 34822683 Free PMC article. Review.
-
Current state and future perspectives of cytochrome P450 enzymes for C-H and C=C oxygenation.Synth Syst Biotechnol. 2022 May 8;7(3):887-899. doi: 10.1016/j.synbio.2022.04.009. eCollection 2022 Sep. Synth Syst Biotechnol. 2022. PMID: 35601824 Free PMC article.
References
-
- Kroetz D. L., Zeldin D. C. 2002. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 13: 273–283. - PubMed
-
- Spector A. A., Fang X., Snyder G. D., Weintraub N. L. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43: 55–90. - PubMed
-
- Roman R. J. 2002. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 82: 131–185. - PubMed
-
- Fer M., Corcos L., Dreano Y., Plee-Gautier E., Salaun J. P., Berthou F., Amet Y. 2008. Cytochromes P450 from family 4 are the main omega hydroxylating enzymes in humans: CYP4F3B is the prominent player in PUFA metabolism. J. Lipid Res. 49: 2379–2389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

