Isoketals form cytotoxic phosphatidylethanolamine adducts in cells
- PMID: 19965577
- PMCID: PMC2853468
- DOI: 10.1194/jlr.M001040
Isoketals form cytotoxic phosphatidylethanolamine adducts in cells
Abstract
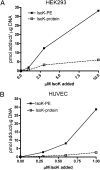
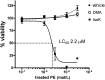
Levuglandins and their stereo- and regio-isomers (termed isolevuglandins or isoketals) are gamma-ketoaldehydes (IsoK) that rapidly react with lysines to form stable protein adducts. IsoK protein adduct levels increase in several pathological conditions including cardiovascular disease. IsoKs can induce ion channel dysfunction and cell death, potentially by adducting to cellular proteins. However, IsoKs also adduct to phosphatidylethanolamine (PE) in vitro, and whether PE adducts form in cells or contribute to the effects of IsoKs is unknown. When radiolabeled IsoK was added to HEK293 cells, 40% of the radiolabel extracted into the chloroform lower phase suggesting the possible formation of PE adducts. We therefore developed methods to measure IsoK-PE adducts in cells. IsoK-PE was quantified by LC/MS/MS after hydrolysis to IsoK-ethanolamine by Streptomyces chromofuscus phospholipase D. In HEK293 and human umbilical vein endothelial cells (HUVEC), IsoK dose-dependently increased PE adduct concentrations to a greater extent than protein adduct. To test the biological significance of IsoK-PE formation, we treated HUVEC with IsoK-PE. IsoK-PE dose dependently induced cytotoxicity (LC(50) 2.2 muM). These results indicate that cellular PE is a significant target of IsoKs, and that formation of PE adducts may mediate some of the biological effects of IsoKs relevant to disease.
Figures







Similar articles
-
Isolevuglandin-modified phosphatidylethanolamine is metabolized by NAPE-hydrolyzing phospholipase D.J Lipid Res. 2013 Nov;54(11):3151-7. doi: 10.1194/jlr.M042556. Epub 2013 Sep 9. J Lipid Res. 2013. PMID: 24018423 Free PMC article.
-
Effects of reactive gamma-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function.FASEB J. 2002 May;16(7):715-7. doi: 10.1096/fj.01-0696fje. Epub 2002 Mar 12. FASEB J. 2002. PMID: 11978738
-
Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin gamma-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry.Nat Protoc. 2007;2(9):2079-91. doi: 10.1038/nprot.2007.298. Nat Protoc. 2007. PMID: 17853863
-
Isolevuglandins (isoLGs) as toxic lipid peroxidation byproducts and their pathogenetic role in human diseases.Free Radic Biol Med. 2021 Jan;162:266-273. doi: 10.1016/j.freeradbiomed.2020.10.024. Epub 2020 Oct 21. Free Radic Biol Med. 2021. PMID: 33099003 Review.
-
Modulation of protein function by isoketals and levuglandins.Subcell Biochem. 2008;49:49-70. doi: 10.1007/978-1-4020-8831-5_2. Subcell Biochem. 2008. PMID: 18751907 Review.
Cited by
-
Isolevuglandin-modified phosphatidylethanolamine is metabolized by NAPE-hydrolyzing phospholipase D.J Lipid Res. 2013 Nov;54(11):3151-7. doi: 10.1194/jlr.M042556. Epub 2013 Sep 9. J Lipid Res. 2013. PMID: 24018423 Free PMC article.
-
Reactive Dicarbonyl Scavenging with 2-Hydroxybenzylamine Improves MASH.Nutrients. 2025 Feb 7;17(4):610. doi: 10.3390/nu17040610. Nutrients. 2025. PMID: 40004939 Free PMC article.
-
The Role of Phosphatidylethanolamine Adducts in Modification of the Activity of Membrane Proteins under Oxidative Stress.Molecules. 2019 Dec 12;24(24):4545. doi: 10.3390/molecules24244545. Molecules. 2019. PMID: 31842328 Free PMC article. Review.
-
Oxidative modification of HDL by lipid aldehydes impacts HDL function.Arch Biochem Biophys. 2022 Nov 15;730:109397. doi: 10.1016/j.abb.2022.109397. Epub 2022 Sep 15. Arch Biochem Biophys. 2022. PMID: 36116503 Free PMC article. Review.
-
Identification of novel bioactive aldehyde-modified phosphatidylethanolamines formed by lipid peroxidation.Free Radic Biol Med. 2012 Sep 15;53(6):1226-38. doi: 10.1016/j.freeradbiomed.2012.07.077. Epub 2012 Aug 4. Free Radic Biol Med. 2012. PMID: 22898174 Free PMC article.
References
-
- Salomon R. G., Miller D. B., Zagorski M. G., Coughlin D. J. 1984. Solvent induced fragmentation of prostaglandin endoperoxides. New aldehyde products from PGH2 and novel intramolecular 1,2-hydride shift during endoperoxide fragmentation in aqueous solution. J. Am. Chem. Soc. 106: 6049–6060.
-
- Salomon R. G., Miller D. B. 1985. Levuglandins: isolation, characterization, and total synthesis of new secoprostanoid products from prostaglandin endoperoxides. Adv. Prostaglandin Thromboxane Leukot. Res. 15: 323–326. - PubMed
-
- Salomon R. G., Sha W., Brame C., Kaur K., Subbanagounder G., O'Neil J., Hoff H. F., Roberts L. J., II 1999. Protein adducts of iso[4]levuglandin E2, a product of the isoprostane pathway, in oxidized low density lipoprotein. J. Biol. Chem. 274: 20271–20280. - PubMed
-
- Salomon R. G., Subbanagounder G., Singh U., O'Neil J., Hoff H. F. 1997. Oxidation of low-density lipoproteins produces levuglandin-protein adducts. Chem. Res. Toxicol. 10: 750–759. - PubMed
-
- Brame C. J., Salomon R. G., Morrow J. D., Roberts L. J., II 1999. Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 274: 13139–13146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

