VLDL lipolysis products increase VLDL fluidity and convert apolipoprotein E4 into a more expanded conformation
- PMID: 19965582
- PMCID: PMC3035491
- DOI: 10.1194/jlr.M000406
VLDL lipolysis products increase VLDL fluidity and convert apolipoprotein E4 into a more expanded conformation
Abstract
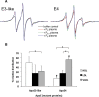
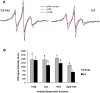
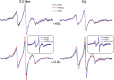
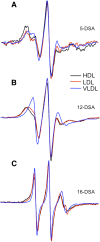
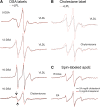
Our previous work indicated that apolipoprotein (apo) E4 assumes a more expanded conformation in the postprandial period. The postprandial state is characterized by increased VLDL lipolysis. In this article, we tested the hypothesis that VLDL lipolysis products increase VLDL particle fluidity, which mediates expansion of apoE4 on the VLDL particle. Plasma from healthy subjects was collected before and after a moderately high-fat meal and incubated with nitroxyl-spin labeled apoE. ApoE conformation was examined by electron paramagnetic resonance spectroscopy using targeted spin probes on cysteines introduced in the N-terminal (S76C) and C-terminal (A241C) domains. Further, we synthesized a novel nitroxyl spin-labeled cholesterol analog, which gave insight into lipoprotein particle fluidity. Our data revealed that the order of lipoprotein fluidity was HDL approximately LDL<VLDL<VLDL+lipoprotein lipase. Moreover, the conformation of apoE4 depended on the lipoprotein fraction: VLDL-associated apoE4 had a more linear conformation than apoE4 associated with LDL or HDL. Further, by changing VLDL fluidity, VLDL lipolysis products significantly altered apoE4 into a more expanded conformation. Our studies indicate that after every meal, VLDL fluidity is increased causing apoE4 associated with VLDL to assume a more expanded conformation, potentially enhancing the pathogenicity of apoE4 in vascular tissue.
Figures
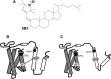

Similar articles
-
Postprandial apoE isoform and conformational changes associated with VLDL lipolysis products modulate monocyte inflammation.PLoS One. 2012;7(11):e50513. doi: 10.1371/journal.pone.0050513. Epub 2012 Nov 28. PLoS One. 2012. PMID: 23209766 Free PMC article.
-
Molecular basis for the differences in lipid and lipoprotein binding properties of human apolipoproteins E3 and E4.Biochemistry. 2010 Dec 28;49(51):10881-9. doi: 10.1021/bi1017655. Epub 2010 Dec 3. Biochemistry. 2010. PMID: 21114327 Free PMC article.
-
Contributions of the carboxyl-terminal helical segment to the self-association and lipoprotein preferences of human apolipoprotein E3 and E4 isoforms.Biochemistry. 2008 Mar 4;47(9):2968-77. doi: 10.1021/bi701923h. Epub 2008 Jan 18. Biochemistry. 2008. PMID: 18201068 Free PMC article.
-
Apolipoprotein E: from cardiovascular disease to neurodegenerative disorders.J Mol Med (Berl). 2016 Jul;94(7):739-46. doi: 10.1007/s00109-016-1427-y. Epub 2016 Jun 9. J Mol Med (Berl). 2016. PMID: 27277824 Free PMC article. Review.
-
[THE LIPOLYSIS IN PHYLOGENETICALLY EARLY LIPOPROTEINS OF LOW DENSITY AND MORE LATER LIPOPROTEINS OF VERY LOW DENSITY: FUNCTION AND DIAGNOSTIC VALUE OF APOE AND APOC-III].Klin Lab Diagn. 2015 Dec;60(12):4-14. Klin Lab Diagn. 2015. PMID: 27032246 Review. Russian.
Cited by
-
Enhanced binding of apolipoprotein A-I variants associated with hypertriglyceridemia to triglyceride-rich particles.Biochemistry. 2011 Mar 29;50(12):2040-7. doi: 10.1021/bi200158b. Epub 2011 Feb 20. Biochemistry. 2011. PMID: 21288012 Free PMC article.
-
Binding of human apoA-I[K107del] variant to TG-rich particles: implications for mechanisms underlying hypertriglyceridemia.J Lipid Res. 2014 Sep;55(9):1876-85. doi: 10.1194/jlr.M047241. Epub 2014 Jun 11. J Lipid Res. 2014. PMID: 24919401 Free PMC article.
-
Increased Binding of Apolipoproteins A-I and E4 to Triglyceride-Rich Lipoproteins is linked to Induction of Hypertriglyceridemia.JSM Atheroscler. 2017;2(2):1026. Epub 2017 Feb 13. JSM Atheroscler. 2017. PMID: 28597004 Free PMC article.
-
Binding of apolipoprotein E inhibits the oligomer growth of amyloid-β peptide in solution as determined by fluorescence cross-correlation spectroscopy.J Biol Chem. 2013 Apr 26;288(17):11628-35. doi: 10.1074/jbc.M112.411900. Epub 2013 Feb 21. J Biol Chem. 2013. PMID: 23430745 Free PMC article.
-
Fluorescence analysis of the lipid binding-induced conformational change of apolipoprotein E4.Biochemistry. 2012 Jul 17;51(28):5580-8. doi: 10.1021/bi300672s. Epub 2012 Jul 3. Biochemistry. 2012. PMID: 22730894 Free PMC article.
References
-
- Davignon J. 2005. Apolipoprotein E and atherosclerosis: beyond lipid effect. Arterioscler. Thromb. Vasc. Biol. 25: 267–269. - PubMed
-
- Weisgraber K. H. 1990. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J. Lipid Res. 31: 1503–1511. - PubMed
-
- Wetterau J. R., Aggerbeck L. P., Rall S. C., Jr., Weisgraber K. H. 1988. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J. Biol. Chem. 263: 6240–6248. - PubMed
-
- Dong L. M., Weisgraber K. H. 1996. Human apolipoprotein E4 domain interaction. Arginine 61 and glutamic acid 255 interact to direct the preference for very low density lipoproteins. J. Biol. Chem. 271: 19053–19057. - PubMed
-
- Saito H., Dhanasekaran P., Baldwin F., Weisgraber K. H., Phillips M. C., Lund-Katz S. 2003. Effects of polymorphism on the lipid interaction of human apolipoprotein E. J. Biol. Chem. 278: 40723–40729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

