MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein
- PMID: 19965586
- PMCID: PMC2853429
- DOI: 10.1194/jlr.M002345
MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann-Pick Type C1 protein
Abstract
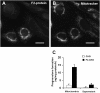
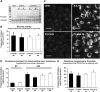
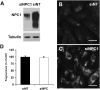
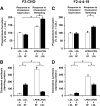
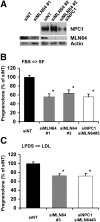
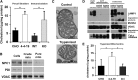
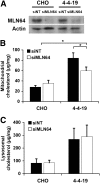
Niemann-Pick Type C (NPC) disease is a fatal, neurodegenerative disorder, caused in most cases by mutations in the late endosomal protein NPC1. A hallmark of NPC disease is endosomal cholesterol accumulation and an impaired cholesterol homeostatic response, which might affect cholesterol transport to mitochondria and, thus, mitochondrial and cellular function. This study aimed to characterize mitochondrial cholesterol homeostasis in NPC disease. Using wild-type and NPC1-deficient Chinese hamster ovary cells, stably transfected with a CYP11A1 complex to assess mitochondrial cholesterol import by pregnenolone production, we show that cholesterol transport to the mitochondrial inner membrane is not affected by loss of NPC1. However, mitochondrial cholesterol content was higher in NPC1-deficient than in wild-type cells. Cholesterol transport to the mitochondrial inner membrane increased markedly upon exposure of cholesterol-deprived cells to lipoproteins, indicating transport of endosomal cholesterol to mitochondria. Reduction of endosomal metastatic lymph node protein 64 (MLN64) by RNA interference decreased cholesterol transport to the mitochondrial inner membrane and reduced mitochondrial cholesterol levels in NPC1-deficient cells, suggesting that MLN64 transported cholesterol to mitochondria even in the absence of NPC1. In summary, this study describes a transport pathway for endosomal cholesterol to mitochondria that requires MLN64, but not NPC1, and that may be responsible for increased mitochondrial cholesterol in NPC disease.
Figures
Comment in
-
STARTing to understand MLN64 function in cholesterol transport.J Lipid Res. 2010 Aug;51(8):2015-7. doi: 10.1194/jlr.E008854. Epub 2010 May 28. J Lipid Res. 2010. PMID: 20511492 Free PMC article. No abstract available.
References
-
- Carstea E. D., Morris J. A., Coleman K. G., Loftus S. K., Zhang D., Cummings C., Gu J., Rosenfeld M. A., Pavan W. J., Krizman D. B., et al. 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 277: 228–231. - PubMed
-
- Naureckiene S., Sleat D. E., Lackland H., Fensom A., Vanier M. T., Wattiaux R., Jadot M., Lobel P. 2000. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 290: 2298–2301. - PubMed
-
- Davies J. P., Ioannou Y. A. 2000. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J. Biol. Chem. 275: 24367–24374. - PubMed
-
- Millard E. E., Gale S. E., Dudley N., Zhang J., Schaffer J. E., Ory D. S. 2005. The sterol-sensing domain of the Niemann-Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J. Biol. Chem. 280: 28581–28590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

