Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes
- PMID: 19965590
- PMCID: PMC2842145
- DOI: 10.1194/jlr.M002782
Glucose stimulates cholesterol 7alpha-hydroxylase gene transcription in human hepatocytes
Abstract
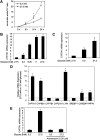
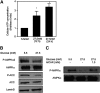
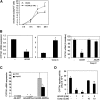
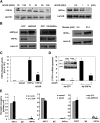
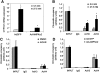
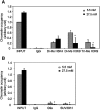
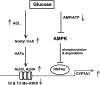
Bile acids play important roles in the regulation of lipid, glucose, and energy homeostasis. Recent studies suggest that glucose regulates gene transcription in the liver. The aim of this study was to investigate the potential role of glucose in regulation of bile acid synthesis in human hepatocytes. High glucose stimulated bile acid synthesis and induced mRNA expression of cholesterol 7alpha-hydroxylase (CYP7A1), the key regulatory gene in bile acid synthesis. Activation of an AMP-activated protein kinase (AMPK) decreased CYP7A1 mRNA, hepatocyte nuclear factor 4alpha (HNF4alpha) protein, and binding to CYP7A1 chromatin. Glucose increased ATP levels to inhibit AMPK and induce HNF4alpha to stimulate CYP7A1 gene transcription. Furthermore, glucose increased histone acetylation and decreased H3K9 di- and tri-methylation in the CYP7A1 chromatin. Knockdown of ATP-citrate lyase, which converts citrate to acetyl-CoA, decreased histone acetylation and attenuated glucose induction of CYP7A1 mRNA expression. These results suggest that glucose signaling also induces CYP7A1 gene transcription by epigenetic regulation of the histone acetylation status. This study uncovers a novel link between hepatic glucose metabolism and bile acid synthesis. Glucose induction of bile acid synthesis may have an important implication in metabolic control of glucose, lipid, and energy homeostasis under normal and diabetic conditions.
Figures

Similar articles
-
A novel role of transforming growth factor beta1 in transcriptional repression of human cholesterol 7alpha-hydroxylase gene.Gastroenterology. 2007 Nov;133(5):1660-9. doi: 10.1053/j.gastro.2007.08.042. Epub 2007 Aug 21. Gastroenterology. 2007. PMID: 17920062
-
Glucagon and cAMP inhibit cholesterol 7alpha-hydroxylase (CYP7A1) gene expression in human hepatocytes: discordant regulation of bile acid synthesis and gluconeogenesis.Hepatology. 2006 Jan;43(1):117-25. doi: 10.1002/hep.20919. Hepatology. 2006. PMID: 16323215
-
A Prospero-related homeodomain protein is a novel co-regulator of hepatocyte nuclear factor 4alpha that regulates the cholesterol 7alpha-hydroxylase gene.J Biol Chem. 2006 Apr 14;281(15):10081-8. doi: 10.1074/jbc.M513420200. Epub 2006 Feb 17. J Biol Chem. 2006. PMID: 16488887 Free PMC article.
-
The pharmacological exploitation of cholesterol 7alpha-hydroxylase, the key enzyme in bile acid synthesis: from binding resins to chromatin remodelling to reduce plasma cholesterol.Pharmacol Ther. 2007 Dec;116(3):449-72. doi: 10.1016/j.pharmthera.2007.08.003. Epub 2007 Sep 7. Pharmacol Ther. 2007. PMID: 17959250 Review.
-
Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP.J Lipid Res. 2002 Apr;43(4):533-43. J Lipid Res. 2002. PMID: 11907135 Review.
Cited by
-
Epigenomic regulation of bile acid metabolism: emerging role of transcriptional cofactors.Mol Cell Endocrinol. 2013 Apr 10;368(1-2):59-70. doi: 10.1016/j.mce.2012.04.008. Epub 2012 May 9. Mol Cell Endocrinol. 2013. PMID: 22579755 Free PMC article. Review.
-
Dysregulated hepatic bile acids collaboratively promote liver carcinogenesis.Int J Cancer. 2016 Oct 15;139(8):1764-75. doi: 10.1002/ijc.30219. Epub 2016 Jun 17. Int J Cancer. 2016. PMID: 27273788 Free PMC article.
-
Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis.Liver Res. 2020 Jun;4(2):47-63. doi: 10.1016/j.livres.2020.05.001. Epub 2020 Jun 3. Liver Res. 2020. PMID: 34290896 Free PMC article.
-
Orphan nuclear receptor oestrogen-related receptor γ (ERRγ) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression.Biochem J. 2015 Sep 1;470(2):181-93. doi: 10.1042/BJ20141494. Epub 2015 Jun 29. Biochem J. 2015. PMID: 26348907 Free PMC article.
-
Macrophage inhibitory cytokine-1 aggravates diet-induced gallstone formation via increased ABCG5/ABCG8 expression.PLoS One. 2023 Jun 13;18(6):e0287146. doi: 10.1371/journal.pone.0287146. eCollection 2023. PLoS One. 2023. PMID: 37310967 Free PMC article.
References
-
- Russell D. W., Setchell K. D. 1992. Bile acid biosynthesis. Biochemistry. 31: 4737–4749 - PubMed
-
- Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. 2009. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 89: 147–191 - PubMed
-
- Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., et al. 2006. Bile acids induce energy expenditure by promoting intra cellular thyroid hormone activation. Nature. 439: 484–489 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

