Participation of ATP7A in macrophage mediated oxidation of LDL
- PMID: 19965596
- PMCID: PMC3035510
- DOI: 10.1194/jlr.M003426
Participation of ATP7A in macrophage mediated oxidation of LDL
Abstract
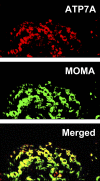
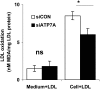
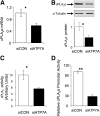
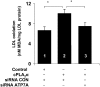
ATP7A primarily functions to egress copper from cells, thereby supplying this cofactor to secreted copper-accepting enzymes. This ATPase has attracted significant attention since the discovery of its mutation leading to human Menkes disease and the demonstration of its distribution in various tissues. Recently, we reported that ATP7A is expressed in the human vasculature. In the present study, we investigated the cellular expression of ATP7A in atherosclerotic lesions of LDL receptor (-/-) mice. Subsequently, we examined the role of ATP7A in regulating the oxidation of LDL in a macrophage cell model. We observed that ATP7A is expressed in atherosclerotic murine aorta and colocalizes with macrophages. To investigate the function of ATP7A, we downregulated ATP7A expression in THP-1 derived macrophages using small interfering RNA (siRNA). ATP7A downregulation attenuated cell-mediated oxidation of LDL. Moreover, downregulation of ATP7A resulted in decreased expression and enzymatic activity of cytosolic phospholipase A(2) alpha (cPLA(2)alpha), a key intracellular enzyme involved in cell-mediated LDL oxidation. In addition, cPLA(2)alpha promoter activity was decreased after downregulation of ATP7A, suggesting that ATP7A transcriptionally regulates cPLA(2)alpha expression. Finally, cPLA(2)alpha overexpression increased LDL oxidation, which was blocked by coadministration of ATP7A siRNA oligonucleotides. These findings suggest a novel mechanism linking ATP7A to cPLA(2)alpha and LDL oxidation, suggesting that this copper transporter could play a previously unrecognized role in the pathogenesis of atherosclerosis.
Figures

Similar articles
-
Human macrophage ATP7A is localized in the trans-Golgi apparatus, controls intracellular copper levels, and mediates macrophage responses to dermal wounds.Inflammation. 2012 Feb;35(1):167-75. doi: 10.1007/s10753-011-9302-z. Inflammation. 2012. PMID: 21336677 Free PMC article.
-
Copper stabilizes the Menkes copper-transporting ATPase (Atp7a) protein expressed in rat intestinal epithelial cells.Am J Physiol Cell Physiol. 2013 Feb 1;304(3):C257-62. doi: 10.1152/ajpcell.00336.2012. Epub 2012 Nov 21. Am J Physiol Cell Physiol. 2013. PMID: 23174565 Free PMC article.
-
A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity.J Biol Chem. 2009 Dec 4;284(49):33949-56. doi: 10.1074/jbc.M109.070201. Epub 2009 Oct 5. J Biol Chem. 2009. PMID: 19808669 Free PMC article.
-
Small amounts of functional ATP7A protein permit mild phenotype.J Trace Elem Med Biol. 2015;31:173-7. doi: 10.1016/j.jtemb.2014.07.022. Epub 2014 Aug 8. J Trace Elem Med Biol. 2015. PMID: 25172213 Review.
-
Copper-transporting ATPases ATP7A and ATP7B: cousins, not twins.J Bioenerg Biomembr. 2007 Dec;39(5-6):403-7. doi: 10.1007/s10863-007-9101-2. J Bioenerg Biomembr. 2007. PMID: 18000748 Review.
Cited by
-
Adipocyte-specific disruption of ATPase copper transporting α in mice accelerates lipoatrophy.Diabetologia. 2019 Dec;62(12):2340-2353. doi: 10.1007/s00125-019-4966-2. Epub 2019 Aug 8. Diabetologia. 2019. PMID: 31396659
-
A New Insight on Atherosclerosis Mechanism and Lipid-Lowering Drugs.Rev Cardiovasc Med. 2025 Mar 5;26(3):25321. doi: 10.31083/RCM25321. eCollection 2025 Mar. Rev Cardiovasc Med. 2025. PMID: 40160588 Free PMC article. Review.
-
Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies.Cell Death Dis. 2023 Feb 11;14(2):105. doi: 10.1038/s41419-023-05639-w. Cell Death Dis. 2023. PMID: 36774340 Free PMC article. Review.
-
Copper ions: The invisible killer of cardiovascular disease (Review).Mol Med Rep. 2024 Nov;30(5):210. doi: 10.3892/mmr.2024.13334. Epub 2024 Sep 20. Mol Med Rep. 2024. PMID: 39301641 Free PMC article. Review.
-
Advances in the understanding of mammalian copper transporters.Adv Nutr. 2011 Mar;2(2):129-37. doi: 10.3945/an.110.000273. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332042 Free PMC article. Review.
References
-
- Schaefer M., Gitlin J. D. 1999. Genetic disorders of membrane transport. IV. Wilson's disease and Menkes disease. Am. J. Physiol. 276: G311–G314. - PubMed
-
- Lutsenko S., et al. 2007. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87: 1011–1046. - PubMed
-
- La Fontaine S., Mercer J. F. 2007. Trafficking of the copper-ATPases, ATP7A and ATP7B: role in copper homeostasis. Arch. Biochem. Biophys. 463: 149–167. - PubMed
-
- Hardman B., et al. 2004. Expression and localization of menkes and Wilson copper transporting ATPases in human placenta. Placenta. 25: 512–517. - PubMed
-
- Paynter J. A., et al. 1994. Expression of the Menkes gene homologue in mouse tissues lack of effect of copper on the mRNA levels. FEBS Lett. 351: 186–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

