Liver X receptor activation promotes macrophage-to-feces reverse cholesterol transport in a dyslipidemic hamster model
- PMID: 19965597
- PMCID: PMC2842160
- DOI: 10.1194/jlr.M001552
Liver X receptor activation promotes macrophage-to-feces reverse cholesterol transport in a dyslipidemic hamster model
Abstract
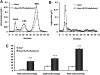
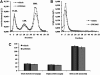
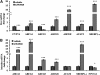
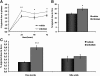
Liver X receptor (LXR) activation promotes reverse cholesterol transport (RCT) in rodents but has major side effects (increased triglycerides and LDL-cholesterol levels) in species expressing cholesteryl ester transfer protein (CETP). In the face of dyslipidemia, it remains unclear whether LXR activation stimulates RCT in CETP species. We therefore used a hamster model made dyslipidemic with a 0.3% cholesterol diet and treated with vehicle or LXR agonist GW3965 (30 mg/kg bid) over 10 days. To investigate RCT, radiolabeled (3)H-cholesterol macrophages or (3)H-cholesteryl oleate-HDL were then injected to measure plasma and feces radioactivity over 72 or 48 h, respectively. The cholesterol-enriched diet increased VLDL-triglycerides and total cholesterol levels in all lipoprotein fractions and strongly increased liver lipids. Overall, GW3965 failed to improve both dyslipidemia and liver steatosis. However, after (3)H-cholesterol labeled macrophage injection, GW3965 treatment significantly increased the (3)H-tracer appearance by 30% in plasma over 72 h, while fecal (3)H-cholesterol excretion increased by 156% (P < 0.001). After (3)H-cholesteryl oleate-HDL injection, GW3965 increased HDL-derived cholesterol fecal excretion by 64% (P < 0.01 vs. vehicle), while plasma fractional catabolic rate remained unchanged. Despite no beneficial effect on dyslipidemia, LXR activation promotes macrophage-to-feces RCT in dyslipidemic hamsters. These results emphasize the use of species with a more human-like lipoprotein metabolism for drug profiling.
Figures
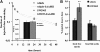
References
-
- Fiévet C., Staels B. 2009. Liver X receptor modulators: effects on lipid metabolism and potential use in the treatment of atherosclerosis. Biochem. Pharmacol. 77: 1316–1327 - PubMed
-
- Naik S. U., Wang X., Da Silva J. S., Jaye M., Macphee C. H., Reilly M. P., Billheimer J. T., Rothblat G. H., Rader D. J. 2006. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 113: 90–97 - PubMed
-
- Calpe-Berdiel L., Rotllan N., Fiévet C., Roig R., Blanco-Vaca F., Escolà-Gil J. C. 2008. Liver X receptor-mediated activation of reverse cholesterol transport from macrophages to feces in vivo requires ABCG5/G8. J. Lipid Res. 49: 1904–1911 - PubMed
-
- Repa J. J., Mangelsdorf D. J. 2002. The liver X receptor gene team: potential new players in atherosclerosis. Nat. Med. 8: 1243–1248 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

