Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid
- PMID: 19965601
- PMCID: PMC2853461
- DOI: 10.1194/jlr.M000257
Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid
Abstract
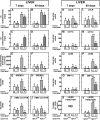
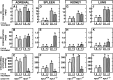
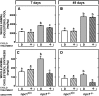
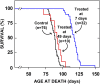
A mutation in NPC1 leads to sequestration of unesterified cholesterol in the late endosomal/lysosomal compartment of every cell culminating in the development of pulmonary, hepatic, and neurodegenerative disease. Acute administration of 2-hydroxypropyl-beta-cyclodextrin (CYCLO) rapidly overcomes this transport defect in both the 7-day-old pup and 49-day-old mature npc1(-/-) mouse, even though this compound is cleared from the body and plasma six times faster in the mature mouse than in the neonatal animal. The liberated cholesterol flows into the cytosolic ester pool, suppresses sterol synthesis, down-regulates SREBP2 and its target genes, and reduces expression of macrophage-associated inflammatory genes. These effects are seen in the liver and brain, as well as in peripheral organs like the spleen and kidney. Only the lung appears to be resistant to these effects. Forty-eight h after CYCLO administration to the 49-day-old animals, fecal acidic, but not neutral, sterol output increases, whole-animal cholesterol burden is reduced, and the hepatic and neurological inflammation is ameliorated. However, lifespan is extended only when the CYCLO is administered to the 7-day-old animals. These studies demonstrate that CYCLO administration acutely reverses the cholesterol transport defect seen in the NPC1 mouse at any age, and this reversal allows the sequestered sterol to be excreted from the body as bile acid.
Figures




Similar articles
-
Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life.Pediatr Res. 2010 Oct;68(4):309-15. doi: 10.1203/PDR.0b013e3181ee4dd2. Pediatr Res. 2010. PMID: 20581737 Free PMC article.
-
Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function.Clin Exp Pharmacol Physiol. 2014 Oct;41(10):780-7. doi: 10.1111/1440-1681.12285. Clin Exp Pharmacol Physiol. 2014. PMID: 25115571 Free PMC article.
-
Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse.Proc Natl Acad Sci U S A. 2009 Feb 17;106(7):2377-82. doi: 10.1073/pnas.0810895106. Epub 2009 Jan 26. Proc Natl Acad Sci U S A. 2009. PMID: 19171898 Free PMC article.
-
Cyclodextrin mediates rapid changes in lipid balance in Npc1-/- mice without carrying cholesterol through the bloodstream.J Lipid Res. 2012 Nov;53(11):2331-42. doi: 10.1194/jlr.M028241. Epub 2012 Aug 14. J Lipid Res. 2012. PMID: 22892156 Free PMC article.
-
Function of the Niemann-Pick type C proteins and their bypass by cyclodextrin.Curr Opin Lipidol. 2011 Jun;22(3):204-9. doi: 10.1097/MOL.0b013e3283453e69. Curr Opin Lipidol. 2011. PMID: 21412152 Review.
Cited by
-
Rescue of an in vitro neuron phenotype identified in Niemann-Pick disease, type C1 induced pluripotent stem cell-derived neurons by modulating the WNT pathway and calcium signaling.Stem Cells Transl Med. 2015 Mar;4(3):230-8. doi: 10.5966/sctm.2014-0127. Epub 2015 Jan 30. Stem Cells Transl Med. 2015. PMID: 25637190 Free PMC article.
-
NPC1 enables cholesterol mobilization during long-term potentiation that can be restored in Niemann-Pick disease type C by CYP46A1 activation.EMBO Rep. 2019 Nov 5;20(11):e48143. doi: 10.15252/embr.201948143. Epub 2019 Sep 18. EMBO Rep. 2019. PMID: 31535451 Free PMC article.
-
Macrocyclic Compounds for Drug and Gene Delivery in Immune-Modulating Therapy.Int J Mol Sci. 2019 Apr 28;20(9):2097. doi: 10.3390/ijms20092097. Int J Mol Sci. 2019. PMID: 31035393 Free PMC article. Review.
-
Sphingolipid lysosomal storage disorders.Nature. 2014 Jun 5;510(7503):68-75. doi: 10.1038/nature13476. Nature. 2014. PMID: 24899306 Review.
-
Impact of loss of SOAT2 function on disease progression in the lysosomal acid lipase-deficient mouse.Steroids. 2018 Feb;130:7-14. doi: 10.1016/j.steroids.2017.11.015. Epub 2017 Dec 13. Steroids. 2018. PMID: 29246491 Free PMC article.
References
-
- Pentchev P. G., Vanier M. T., Suzuki K., Patterson M. C. 1995. Niemann-Pick disease type C: a cellular cholesterol lipidosis. The Metabolic and Molecular Bases of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Stanbury J. B., Wyngaarden J. B., Fredrickson D. S., McGraw-Hill, New York: 2625–2639.
-
- Patterson M. C. 2003. A riddle wrapped in a mystery: understanding Niemann-Pick disease, type C. Neurologist. 9: 301–310. - PubMed
-
- Vanier M. T., Millat G. 2003. Niemann-Pick disease type C. Clin. Genet. 64: 269–281. - PubMed
-
- Manabe T., Yamane T., Higashi Y., Pentchev P. G., Suzuki K. 1995. Ultrastructural changes in the lung in Niemann-Pick type C mouse. Virchows Arch. 427: 77–83. - PubMed
-
- Neufeld E. B., Wastney M., Patel S., Suresh S., Cooney A. M., Dwyer N. K., Roff C. F., Ohno K., Morris J. A., Carstea E. D., et al. 1999. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 274: 9627–9635. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

