Asymmetric synthesis and structure elucidation of a glycerophospholipid from Mycobacterium tuberculosis
- PMID: 19965610
- PMCID: PMC2853428
- DOI: 10.1194/jlr.M001982
Asymmetric synthesis and structure elucidation of a glycerophospholipid from Mycobacterium tuberculosis
Abstract
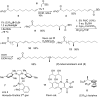
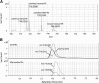
A glycerophospholipid (1-O-tuberculostearoyl-2-O-palmitoyl-sn-glycero-3-phosphoethanolamine) from Mycobacterium tuberculosis was isolated from the reference strain H37Rv. The molecular structure of this tuberculostearoyl [(R)-10-methyloctadecyl] and palmitoyl containing phosphatidylethanolamine (PE) has been resolved. The substitution pattern on the glycerol backbone could be determined by comparison of the isolate to the two synthetically prepared regioisomers. MS/MS analysis was used to determine its molecular structure. Production of this synthetic version of mycobacterial PE in high yield, with a stereochemically correct and pathogen-specific fatty acyl group, can be used as a standard in LC-MS based lipidomic analyses to detect trace amounts of mycobacterial PE in human blood, sputum, or tissues as a marker of infection by mycobacteria.
Figures



References
-
- Vance J. E. 2008. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49: 1377–1387. - PubMed
-
- Nicolaou K. C., Snyder S. A. 2005. Chasing molecules that were never there: misassigned natural products and the role of chemical synthesis in modern structure elucidation. Agnew. Chem. Int. Ed. 44: 1012–1044. - PubMed
-
- Smith P. B., Snyder A. P., Harden C. S. 1995. Characterization of bacterial phospholipids by electrospray ionization tandem mass spectrometry. Anal. Chem. 67: 1824–1830. - PubMed
-
- Cabrera G. M., Fernández Murga M. L., Font de Valdez G., Seldes A. M. 2000. Direct analysis by electrospray ionisation tandem mass spectrometry of mixtures of phosphatidyldiacylglycerols from Lactobacillus. J. Am. Soc. Mass Spectrom. 35: 1452–1459. - PubMed
-
- Jensen N. J., Tomer K. B., Gross M. L. 1987. FAB MS/MS for phosphatidylinostitol,-glycerol,-ethanolamine and other complex phospholipids. Lipids. 22: 480–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

