Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing
- PMID: 19965612
- PMCID: PMC2853460
- DOI: 10.1194/jlr.M000059
Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing
Abstract
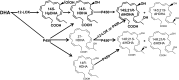
Chronic wounds remain a medical challenge, where well-coordinated cellular and molecular processes required by optimal healing are impaired by diabetes, aging, or other diseases. In determining mechanisms that regulate wound healing, we found that wounding induced formation of novel endogenous 14S,21S-dihydroxy-docosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acids (14S,21S-diHDHA);14R,21R-diHDHA; 14S,21R-diHDHA; and/or 14R,21S-diHDHA. 12-lipoxygenase and cytochrome P450 catalysis in tandem converted docosahexaenoic acid to 14S,21R-diHDHA and 14S,21S-diHDHA through the intermediacy of 14S-HDHA; P450 also converted 14R-HDHA to novel 14R,21R-diHDHA and 14R,21S-diHDHA. Macrophages function as the combination of 12-lipoxgenase and P450 to generate these 14,21-diHDHA stereoisomers, as well as their intermediates 14S-HDHA, 14R-HDHA, and 21-HDHA. The structure and formation pathways of 14,21-diHDHA stereoisomers were further confirmed by macrophage biosynthesis of 14,21-diHDHA-21,22,22,22-d(4) stereoisomers, 14S-HDHA-d(5), 14R-HDHA-d(5), and 21-HDHA-d(4) from DHA-21,21,22,22,22-d(5). We found that 14S,21-diHDHA and 14R,21-diHDHA enhanced wound closure, reepithelialization, granulation tissue growth, and capillary vasculature formation of murine wounds. 14S,21-diHDHA and 14R,21-diHDHA produced by macrophages may partially represent the molecular mechanisms for macrophage pro-healing function. Taken together, 14,21-dihydroxy-DHA stereoisomers and their formation pathways may represent a novel mechanism in the orchestration of wound healing processes, which may provide new insight for developing novel therapeutic modalities that counteract impairments to wound healing.
Figures



References
-
- Martin P. 1997. Wound healing–aiming for perfect skin regeneration. Science. 276: 75–81. - PubMed
-
- Falanga V. 2005. Wound healing and its impairment in the diabetic foot. Lancet. 366: 1736–1743. - PubMed
-
- Serhan C. N., Clish C. B., Brannon J., Colgan S. P., Chiang N., Gronert K. 2000. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 192: 1197–1204. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

