Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion
- PMID: 19965634
- PMCID: PMC2920226
- DOI: 10.1182/blood-2009-07-235382
Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion
Abstract
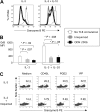
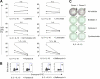
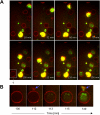
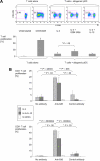
Human plasmacytoid dendritic cells (pDCs) are crucially involved in the modulation of adaptive T-cell responses in the course of neoplastic, viral, and autoimmune disorders. In several of these diseases elevated extracellular levels of the serine protease granzyme B (GrB) are observed. Here we demonstrate that human pDCs can be an abundant source of GrB and that such GrB(+) pDCs potently suppress T-cell proliferation in a GrB-dependent, perforin-independent manner, a process reminiscent of regulatory T cells. Moreover, we show that GrB expression is strictly regulated on a transcriptional level involving Janus kinase 1 (JAK1), signal transducer and activator of transcription 3 (STAT3), and STAT5 and that interleukin-3 (IL-3), a cytokine secreted by activated T cells, plays a central role for GrB induction. Moreover, we find that the immunosuppressive cytokine IL-10 enhances, while Toll-like receptor agonists and CD40 ligand strongly inhibit, GrB secretion by pDCs. GrB-secreting pDCs may play a regulatory role for immune evasion of tumors, antiviral immune responses, and autoimmune processes. Our results provide novel information about the complex network of pDC-T-cell interactions and may contribute to an improvement of prophylactic and therapeutic vaccinations.
Figures



References
-
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8(8):594–606. - PubMed
-
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5(12):1219–1226. - PubMed
-
- Waldner H. The role of innate immune responses in autoimmune disease development. Autoimmun Rev. 2009;8(5):400–404. - PubMed
-
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

