Regulating human Th17 cells via differential expression of IL-1 receptor
- PMID: 19965648
- PMCID: PMC2810985
- DOI: 10.1182/blood-2009-08-236521
Regulating human Th17 cells via differential expression of IL-1 receptor
Abstract
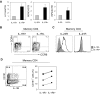
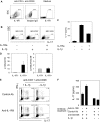
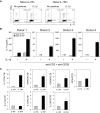
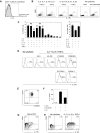
In humans, interleukin-1beta (IL-1beta) has been suggested as an essential cytokine for developing IL-17- or IL-17A-producing CD4(+) T helper 17 (Th17) cells. However, little is known about the relationship of IL-1 receptor expression and Th17 cell differentiation. We report here the presence of 2 distinct CD4(+) T-cell populations with and without expression of IL-1RI that correlates with the capacity to produce IL-17 in naive and memory CD4(+) T cells of human peripheral blood. IL-1RI(+) memory CD4(+) T cells had increased gene expression of IL17, RORC, and IRF4 even before T-cell receptor triggering, indicating that the effect of IL-1beta is programmed in these cells via IL-1RI. Although CD4(+) T cells from umbilical cord blood did not express IL-1RI, the cytokines IL-7, IL-15, and transforming growth factor-beta (TGF-beta) up-regulated IL-1RI expression on naive CD4(+) T cells, suggesting that IL-1RI(+) naive CD4(+) T cells develop in periphery. Furthermore, IL-17 production from the cytokine-treated naive CD4(+) T cells was induced by IL-1beta and this induction was blocked by IL-1R antagonist. These results indicate that human Th17 cell differentiation is regulated via differential expression of IL-1RI, which is controlled by IL-7 and IL-15.
Figures



Similar articles
-
Ex vivo IL-1 receptor type I expression in human CD4+ T cells identifies an early intermediate in the differentiation of Th17 from FOXP3+ naive regulatory T cells.J Immunol. 2011 Nov 15;187(10):5196-202. doi: 10.4049/jimmunol.1101742. Epub 2011 Oct 12. J Immunol. 2011. PMID: 21998454
-
Prostaglandin E2 and IL-23 plus IL-1β differentially regulate the Th1/Th17 immune response of human CD161(+) CD4(+) memory T cells.Clin Transl Sci. 2011 Aug;4(4):268-73. doi: 10.1111/j.1752-8062.2011.00300.x. Clin Transl Sci. 2011. PMID: 21884514 Free PMC article.
-
TGF-β enhanced IL-21-induced differentiation of human IL-21-producing CD4+ T cells via Smad3.PLoS One. 2013 May 31;8(5):e64612. doi: 10.1371/journal.pone.0064612. Print 2013. PLoS One. 2013. PMID: 23741351 Free PMC article.
-
IL-1β strikingly enhances antigen-driven CD4 and CD8 T-cell responses.Cold Spring Harb Symp Quant Biol. 2013;78:117-24. doi: 10.1101/sqb.2013.78.021246. Epub 2013 Oct 3. Cold Spring Harb Symp Quant Biol. 2013. PMID: 24092469 Review.
-
Modulation of autoimmune diseases by interleukin (IL)-17 producing regulatory T helper (Th17) cells.Indian J Med Res. 2013 Nov;138(5):591-4. Indian J Med Res. 2013. PMID: 24434314 Free PMC article. Review.
Cited by
-
IL-1 and T Helper Immune Responses.Front Immunol. 2013 Jul 15;4:182. doi: 10.3389/fimmu.2013.00182. eCollection 2013. Front Immunol. 2013. PMID: 23874332 Free PMC article.
-
Lung cancer: a classic example of tumor escape and progression while providing opportunities for immunological intervention.Clin Dev Immunol. 2012;2012:160724. doi: 10.1155/2012/160724. Epub 2012 Jul 29. Clin Dev Immunol. 2012. PMID: 22899945 Free PMC article. Review.
-
Chemokine positioning determines mutually exclusive roles for their receptors in extravasation of pathogenic human T cells.bioRxiv [Preprint]. 2023 Feb 13:2023.01.25.525561. doi: 10.1101/2023.01.25.525561. bioRxiv. 2023. PMID: 36789428 Free PMC article. Preprint.
-
IL-1β Induces the Rapid Secretion of the Antimicrobial Protein IL-26 from Th17 Cells.J Immunol. 2019 Aug 15;203(4):911-921. doi: 10.4049/jimmunol.1900318. Epub 2019 Jun 24. J Immunol. 2019. PMID: 31235553 Free PMC article.
-
Anakinra-Loaded Sphingomyelin Nanosystems Modulate In Vitro IL-1-Dependent Pro-Tumor Inflammation in Pancreatic Cancer.Int J Mol Sci. 2024 Jul 24;25(15):8085. doi: 10.3390/ijms25158085. Int J Mol Sci. 2024. PMID: 39125655 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

