CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus
- PMID: 19965655
- PMCID: PMC2837318
- DOI: 10.1182/blood-2009-08-237784
CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus
Abstract
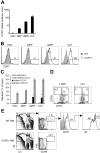
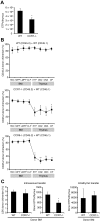
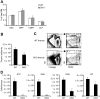
T lymphopoiesis requires settling of the thymus by bone marrow-derived precursors throughout adult life. Progenitor entry into the thymus is selective, but the molecular basis of this selectivity is incompletely understood. The chemokine receptor CCR9 has been demonstrated to be important in this process. However, progenitors lacking CCR9 can still enter the thymus, suggesting a role for additional molecules. Here we report that the chemokine receptor CCR7 is also required for efficient thymic settling. CCR7 is selectively expressed on bone marrow progenitors previously shown to have the capacity to settle the thymus, and CCR7(-/-) progenitors are defective in settling the thymus. We further demonstrate that CCR7 sustains thymic settling in the absence of CCR9. Mice deficient for both CCR7 and CCR9 have severe reductions in the number of early thymic progenitors, and in competitive assays CCR7(-/-)CCR9(-/-) double knockout progenitors are almost completely restricted from thymic settling. However, these mice possess near-normal thymic cellularity. Compensatory expansion of intrathymic populations can account for at least a part of this recovery. Together our results illustrate the critical role of chemokine receptor signaling in thymic settling and help to clarify the cellular identity of the physiologic thymic settling progenitors.
Figures

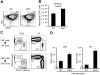
Comment in
-
CCR7/CCR9: knockin' on the thymus door.Blood. 2010 Mar 11;115(10):1861-2. doi: 10.1182/blood-2010-01-258848. Blood. 2010. PMID: 20223929 No abstract available.
Similar articles
-
CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus.Blood. 2010 Mar 11;115(10):1906-12. doi: 10.1182/blood-2009-07-235721. Epub 2009 Dec 29. Blood. 2010. PMID: 20040757
-
Delivery of progenitors to the thymus limits T-lineage reconstitution after bone marrow transplantation.Blood. 2011 Aug 18;118(7):1962-70. doi: 10.1182/blood-2010-12-324954. Epub 2011 Jun 9. Blood. 2011. PMID: 21659540 Free PMC article.
-
Clonal analysis reveals uniformity in the molecular profile and lineage potential of CCR9(+) and CCR9(-) thymus-settling progenitors.J Immunol. 2011 May 1;186(9):5227-35. doi: 10.4049/jimmunol.1002686. Epub 2011 Mar 18. J Immunol. 2011. PMID: 21421850 Free PMC article.
-
Trafficking to the thymus.Curr Top Microbiol Immunol. 2014;373:87-111. doi: 10.1007/82_2013_324. Curr Top Microbiol Immunol. 2014. PMID: 23624945 Review.
-
Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis.Immunol Rev. 2006 Feb;209:47-57. doi: 10.1111/j.0105-2896.2006.00350.x. Immunol Rev. 2006. PMID: 16448533 Review.
Cited by
-
CXCR4-related increase of circulating human lymphoid progenitors after allogeneic hematopoietic stem cell transplantation.PLoS One. 2014 Mar 12;9(3):e91492. doi: 10.1371/journal.pone.0091492. eCollection 2014. PLoS One. 2014. PMID: 24621606 Free PMC article.
-
Cyp1b1-mediated suppression of lymphoid progenitors in bone marrow by polycyclic aromatic hydrocarbons coordinately impacts spleen and thymus: a selective role for the Ah Receptor.Pharmacol Res Perspect. 2016 Jul 29;4(4):e00245. doi: 10.1002/prp2.245. eCollection 2016 Aug. Pharmacol Res Perspect. 2016. PMID: 28116098 Free PMC article.
-
Heparan sulfate is essential for thymus growth.J Biol Chem. 2021 Jan-Jun;296:100419. doi: 10.1016/j.jbc.2021.100419. Epub 2021 Feb 15. J Biol Chem. 2021. PMID: 33600795 Free PMC article.
-
Colonization and effector functions of innate lymphoid cells in mucosal tissues.Microbes Infect. 2016 Oct;18(10):604-614. doi: 10.1016/j.micinf.2016.06.005. Epub 2016 Jun 27. Microbes Infect. 2016. PMID: 27365193 Free PMC article. Review.
-
Lymphotoxin β Receptor Controls T Cell Progenitor Entry to the Thymus.J Immunol. 2016 Oct 1;197(7):2665-72. doi: 10.4049/jimmunol.1601189. Epub 2016 Aug 22. J Immunol. 2016. PMID: 27549174 Free PMC article.
References
-
- Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life: a study in parabiotic mice. J Immunol. 1992;148(6):1604–1612. - PubMed
-
- Scollay R, Smith J, Stauffer V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol Rev. 1986;91:129–157. - PubMed
-
- Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

