Immunization with host-type CD8{alpha}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner
- PMID: 19965670
- PMCID: PMC2810989
- DOI: 10.1182/blood-2009-06-229708
Immunization with host-type CD8{alpha}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner
Abstract
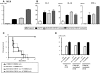
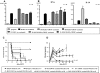
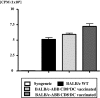
Little is known about the role of active immunization in suppressing undesirable immune responses. Because CD8alpha(+) dendritic cells (DCs) suppress certain immune responses, we tested the hypothesis that immunization of donors with host-derived CD8alpha(+) DCs will reduce host-specific donor T-cell responses. BALB/c T cells from the animals that were immunized with B6 CD8alpha(+) DCs demonstrated, in vitro and in vivo, significantly reduced proliferation and secretion of inflammatory cytokines but showed enhanced secretion of interleukin-10 (IL-10). The responses against third-party and model antigens were preserved demonstrating antigen specificity. The in vivo relevance was further demonstrated by the reduction on graft-versus-host disease (GVHD) in both a major histocompatibility complex-mismatched clinically relevant BALB/c --> B6 model and major histocompatibility complex-matched, minor-mismatched C3H.SW --> B6 model of GVHD. Immunization of the donors that were deficient in IL-10 (IL-10(-/-)) or with CD8alpha(+) DCs from B6 class II (class II(-/-)) failed to reduce T-cell responses, demonstrating (1) a critical role for secretion of IL-10 by donor T cells and (2) a direct contact between the T cells and the CD8alpha(+) DCs. Together, these data may represent a novel strategy for reducing GVHD and suggest a broad counterintuitive role for vaccination strategies in mitigating undesirable immune responses in an antigen-specific manner.
Figures
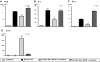
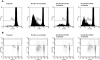

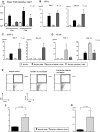
References
-
- Nossal GJ. Host immunobiology and vaccine development. Lancet. 1997;350(9087):1316–1319. - PubMed
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
-
- Appelbaum FR. Haematopoietic cell transplantation as immunotherapy. Nature. 2001;411(6835):385–389. - PubMed
-
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

