gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis
- PMID: 19965672
- PMCID: PMC2845896
- DOI: 10.1182/blood-2009-07-233031
gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis
Abstract
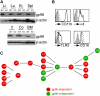
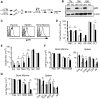
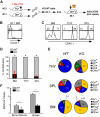
Integrins contribute to lymphopoiesis, whereas Toll-like receptors (TLRs) facilitate the myeloid replenishment during inflammation. The combined role of TLRs and integrin on hematopoiesis remains unclear. gp96 (grp94, HSP90b1) is an endoplasmic reticulum master chaperone for multiple TLRs. We report herein that gp96 is also essential for expression of 14 hematopoietic system-specific integrins. Genetic deletion of gp96 thus enables us to determine the collective roles of gp96, integrins, and TLRs in hematopoiesis. We found that gp96-null hematopoietic stem cells could support long-term myelopoiesis. B- and T-cell development, however, was severely compromised with transitional block from pro-B to pre-B cells and the inability of thymocytes to develop beyond the CD4(-)CD8(-) stage. These defects were cell-intrinsic and could be recapitulated on bone marrow stromal cell culture. Furthermore, defective lymphopoiesis correlated strongly with failure of hematopoietic progenitors to form close contact with stromal cell niche and was not the result of the defect in the assembly of antigen receptor or interleukin-7 signaling. These findings define gp96 as the only known molecular chaperone to specifically regulate T- and B-cell development.
Figures




Comment in
-
Chaperoning the lympho-stromal dance.Blood. 2010 Mar 25;115(12):2334-5. doi: 10.1182/blood-2009-12-254920. Blood. 2010. PMID: 20339105 No abstract available.
References
-
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4(4):E83–E90. - PubMed
-
- Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for alpha4 integrins during fetal and adult hematopoiesis. Cell. 1996;85(7):997–1008. - PubMed
-
- Arroyo AG, Yang JT, Rayburn H, Hynes RO. Alpha4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity. 1999;11(5):555–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

