Granzyme B is not required for regulatory T cell-mediated suppression of graft-versus-host disease
- PMID: 19965675
- PMCID: PMC2832813
- DOI: 10.1182/blood-2009-07-233676
Granzyme B is not required for regulatory T cell-mediated suppression of graft-versus-host disease
Abstract
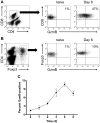
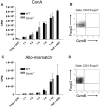
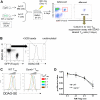
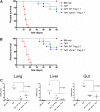
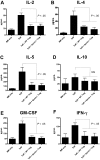
Regulatory T (T(reg)) cells can suppress a wide variety of immune responses, including antitumor and alloimmune responses. The mechanisms by which T(reg) cells mediate their suppressive effects depend on the context of their activation. We previously reported that granzyme B is important for T(reg) cell-mediated suppression of antitumor immune responses. We therefore hypothesized that granzyme B may likewise be important for suppression of graft-versus-host disease (GVHD). We found that allogeneic mismatch induces the expression of granzyme B in mixed lymphocyte reactions and in a model of graft-versus-host disease (GVHD). However, wild-type and granzyme B-deficient T(reg) cells were equally able to suppress effector T (T(eff)) cell proliferation driven by multiple stimuli, including allogeneicantigen-presenting cells. Surprisingly, adoptive transfer of granzyme B-deficient T(reg) cells prevented GVHD lethality, suppressed serum cytokine production in vivo, and prevented target organ damage. These data contrast strikingly with our previous study, which demonstrated that granzyme B plays a nonredundant role in T(reg) cell-mediated suppression of antitumor responses. Taken together, these findings suggest that targeting specific T(reg) cell-suppressive mechanisms, such as granzyme B, may be therapeutically beneficial for segregating GVHD and graft-versus-tumor immune responses.
Figures


Comment in
-
Separation of GVHD and GVL.Blood. 2010 Mar 4;115(9):1666-7. doi: 10.1182/blood-2009-11-254946. Blood. 2010. PMID: 20203273 No abstract available.
References
-
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset: I. Evidence for the active participation of T cells in natural self-tolerance: deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72–87. - PMC - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
-
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. - PubMed
-
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

