Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction
- PMID: 19965872
- PMCID: PMC2823547
- DOI: 10.1074/jbc.M109.056523
Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction
Abstract
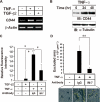
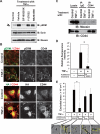
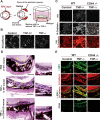
Aberrant epithelial-mesenchymal transition (EMT) is involved in development of fibrotic disorders and cancer invasion. Alterations of cell-extracellular matrix interaction also contribute to those pathological conditions. However, the functional interplay between EMT and cell-extracellular matrix interactions remains poorly understood. We now show that the inflammatory mediator tumor necrosis factor-alpha (TNF-alpha) induces the formation of fibrotic foci by cultured retinal pigment epithelial cells through activation of transforming growth factor-beta (TGF-beta) signaling in a manner dependent on hyaluronan-CD44-moesin interaction. TNF-alpha promoted CD44 expression and moesin phosphorylation by protein kinase C, leading to the pericellular interaction of hyaluronan and CD44. Formation of the hyaluronan-CD44-moesin complex resulted in both cell-cell dissociation and increased cellular motility through actin remodeling. Furthermore, this complex was found to be associated with TGF-beta receptor II and clathrin at actin microdomains, leading to activation of TGF-beta signaling. We established an in vivo model of TNF-alpha-induced fibrosis in the mouse eye, and such ocular fibrosis was attenuated in CD44-null mice. The production of hyaluronan and its interaction with CD44, thus, play an essential role in TNF-alpha-induced EMT and are potential therapeutic targets in fibrotic disorders.
Figures





References
-
- Thiery J. P. (2002) Nat. Rev. Cancer 2, 442–454 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

