Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis
- PMID: 19965930
- PMCID: PMC5428123
- DOI: 10.1210/me.2009-0236
Antiinflammatory properties of a plant-derived nonsteroidal, dissociated glucocorticoid receptor modulator in experimental autoimmune encephalomyelitis
Abstract
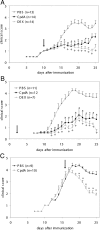
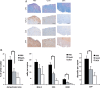
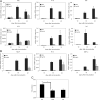
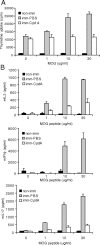
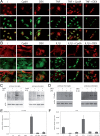
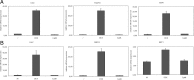
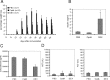
Compound A (CpdA), a plant-derived phenyl aziridine precursor, was recently characterized as a fully dissociated nonsteroidal antiinflammatory agent, acting via activation of the glucocorticoid receptor, thereby down-modulating nuclear factor-kappaB-mediated transactivation, but not supporting glucocorticoid response element-driven gene expression. The present study demonstrates the effectiveness of CpdA in inhibiting the disease progress in experimental autoimmune encephalomyelitis (EAE), a well-characterized animal model of multiple sclerosis. CpdA treatment of mice, both early and at the peak of the disease, markedly suppressed the clinical symptoms of EAE induced by myelin oligodendrocyte glycoprotein peptide immunization. Attenuation of the clinical symptoms of EAE by CpdA was accompanied by reduced leukocyte infiltration in the spinal cord, reduced expression of inflammatory cytokines and chemokines, and reduced neuronal damage and demyelination. In vivo CpdA therapy suppressed the encephalogenicity of myelin oligodendrocyte glycoprotein peptide-specific T cells. Moreover, CpdA was able to inhibit TNF- and lipopolysaccharide-induced nuclear factor-kappaB activation in primary microglial cells in vitro, in a differential mechanistic manner as compared with dexamethasone. Finally, in EAE mice the therapeutic effect of CpdA, in contrast to that of dexamethasone, occurred in the absence of hyperinsulinemia and in the absence of a suppressive effect on the hypothalamic-pituitary-adrenal axis. Based on these results, we propose CpdA as a compound with promising antiinflammatory characteristics useful for therapeutic intervention in multiple sclerosis and other neuroinflammatory diseases.
Figures
Similar articles
-
Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis.PLoS One. 2009 Dec 7;4(12):e8202. doi: 10.1371/journal.pone.0008202. PLoS One. 2009. PMID: 19997594 Free PMC article.
-
A fully dissociated compound of plant origin for inflammatory gene repression.Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):15827-32. doi: 10.1073/pnas.0505554102. Epub 2005 Oct 21. Proc Natl Acad Sci U S A. 2005. PMID: 16243974 Free PMC article.
-
A dissociated glucocorticoid receptor modulator reduces airway hyperresponsiveness and inflammation in a mouse model of asthma.J Immunol. 2012 Apr 1;188(7):3478-87. doi: 10.4049/jimmunol.1004227. Epub 2012 Mar 5. J Immunol. 2012. PMID: 22393156
-
Novel disease-modifying anti-rheumatic drug iguratimod suppresses chronic experimental autoimmune encephalomyelitis by down-regulating activation of macrophages/microglia through an NF-κB pathway.Sci Rep. 2018 Jan 31;8(1):1933. doi: 10.1038/s41598-018-20390-5. Sci Rep. 2018. PMID: 29386552 Free PMC article.
-
Thymosins in multiple sclerosis and its experimental models: moving from basic to clinical application.Mult Scler Relat Disord. 2019 Jan;27:52-60. doi: 10.1016/j.msard.2018.09.035. Epub 2018 Oct 2. Mult Scler Relat Disord. 2019. PMID: 30317071 Free PMC article. Review.
Cited by
-
Disease- and treatment-associated acquired glucocorticoid resistance.Endocr Connect. 2018 Dec;7(12):R328-R349. doi: 10.1530/EC-18-0421. Endocr Connect. 2018. PMID: 30352419 Free PMC article. Review.
-
Compound A Increases Cell Infiltration in Target Organs of Acute Graft-versus-Host Disease (aGVHD) in a Mouse Model.Molecules. 2021 Jul 12;26(14):4237. doi: 10.3390/molecules26144237. Molecules. 2021. PMID: 34299512 Free PMC article.
-
Ligand-dependent genomic function of glucocorticoid receptor in triple-negative breast cancer.Nat Commun. 2015 Sep 16;6:8323. doi: 10.1038/ncomms9323. Nat Commun. 2015. PMID: 26374485 Free PMC article.
-
Role of microglia in CNS autoimmunity.Clin Dev Immunol. 2013;2013:208093. doi: 10.1155/2013/208093. Epub 2013 Jun 12. Clin Dev Immunol. 2013. PMID: 23840238 Free PMC article. Review.
-
Selective modulation through the glucocorticoid receptor ameliorates muscle pathology in mdx mice.J Pathol. 2013 Oct;231(2):223-35. doi: 10.1002/path.4231. J Pathol. 2013. PMID: 23794417 Free PMC article.
References
-
- Hemmer B, Archelos JJ, Hartung HP2002. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci 3:291–301 - PubMed
-
- McFarland HF, Martin R2007. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol 8:913–919 - PubMed
-
- Steinman L1996. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302 - PubMed
-
- Baxter AG2007. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol 7:904–912 - PubMed
-
- Farina C, Aloisi F, Meinl E2007. Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

