Molecular biological effects of selective neuronal nitric oxide synthase inhibition in ovine lung injury
- PMID: 19965980
- PMCID: PMC2838669
- DOI: 10.1152/ajplung.00147.2009
Molecular biological effects of selective neuronal nitric oxide synthase inhibition in ovine lung injury
Abstract
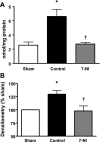
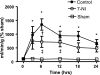
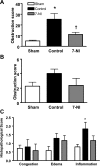
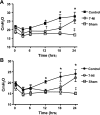
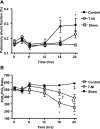
Neuronal nitric oxide synthase is critically involved in the pathogenesis of acute lung injury resulting from combined burn and smoke inhalation injury. We hypothesized that 7-nitroindazole, a selective neuronal nitric oxide synthase inhibitor, blocks central molecular mechanisms involved in the pathophysiology of this double-hit insult. Twenty-five adult ewes were surgically prepared and randomly allocated to 1) an uninjured, untreated sham group (n = 7), 2) an injured control group with no treatment (n = 7), 3) an injury group treated with 7-nitroindazole from 1-h postinjury to the remainder of the 24-h study period (n = 7), or 4) a sham-operated group subjected only to 7-nitroindazole to judge the effects in health. The combination injury was associated with twofold increased activity of neuronal nitric oxide synthase and oxidative/nitrosative stress, as indicated by significant increases in plasma nitrate/nitrite concentrations, 3-nitrotyrosine (an indicator of peroxynitrite formation), and malondialdehyde lung tissue content. The presence of systemic inflammation was evidenced by twofold, sixfold, and threefold increases in poly(ADP-ribose) polymerase, IL-8, and myeloperoxidase lung tissue concentrations, respectively (each P < 0.05 vs. sham). These molecular changes were linked to tissue damage, airway obstruction, and pulmonary shunting with deteriorated gas exchange. 7-Nitroindazole blocked, or at least attenuated, all these pathological changes. Our findings suggest 1) that nitric oxide formation derived from increased neuronal nitric oxide synthase activity represents a pivotal reactive agent in the patho-physiology of combined burn and smoke inhalation injury and 2) that selective neuronal nitric oxide synthase inhibition represents a goal-directed approach to attenuate the degree of injury.
Figures
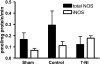


References
-
- Adaikalakoteswari A, Rema M, Mohan V, Balasubramanyam M. Oxidative DNA damage and augmentation of poly(ADP-ribose) polymerase/nuclear factor-kappa B signaling in patients with type 2 diabetes and microangiopathy. Int J Biochem Cell Biol 39: 1673–1684, 2007 - PubMed
-
- Bakondi E, Bai P, Szabo EE, Hunyadi J, Gergely P, Szabo C, Virag L. Detection of poly(ADP-ribose) polymerase activation in oxidatively stressed cells and tissues using biotinylated NAD substrate. J Histochem Cytochem 50: 91–98, 2002 - PubMed
-
- Barth E, Radermacher P, Szabo C. The world according to poly(ADP-ribose) polymerase (PARP)–update 2006. Intensive Care Med 32: 1470–1474, 2006 - PubMed
-
- Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann NY Acad Sci 150: 874–894, 1968 - PubMed
-
- Beller CJ, Radovits T, Kosse J, Gero D, Szabo C, Szabo G. Activation of the peroxynitrite-poly(adenosine diphosphate-ribose) polymerase pathway during neointima proliferation: a new target to prevent restenosis after endarterectomy. J Vasc Surg 43: 824–830, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

