Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of the tdh and trh genes of Vibrio parahaemolyticus and related Vibrio species
- PMID: 19966027
- PMCID: PMC2812988
- DOI: 10.1128/AEM.02284-09
Development of a loop-mediated isothermal amplification assay for sensitive and rapid detection of the tdh and trh genes of Vibrio parahaemolyticus and related Vibrio species
Abstract
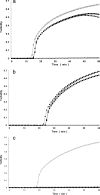
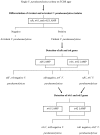
Thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) are the major virulence determinants of Vibrio parahaemolyticus. TRH is further differentiated into TRH1 and TRH2 on the basis of genetic and phenotypic differences. We developed a novel and highly specific loop-mediated isothermal amplification (LAMP) assay for sensitive and rapid detection of the tdh, trh1, and trh2 genes of V. parahaemolyticus. The LAMP assay was designed for both combined and individual detection of the tdh, trh1, and trh2 genes and combined detection of the trh1 and trh2 genes. Our results showed that it gave the same results as DNA probes and conventional PCR assays for 125 strains of V. parahaemolyticus, 3 strains of Grimontia hollisae, and 2 strains of Vibrio mimicus carrying the tdh, trh1, and trh2 genes in various combinations. No LAMP products were detected for any of the 20 bacterial strains lacking the tdh, trh1, and trh2 genes. The sensitivities of the LAMP assay for detection of tdh-, trh1-, and trh2-carrying V. parahaemolyticus strains in spiked shrimp samples were 0.8, 21.3, and 5.0 CFU per LAMP reaction tube, respectively. Starting with DNA extraction from a single colony and from spiked shrimp samples, the LAMP assay required only 27 to 60 min and less than 80 min, respectively. This is the first report of a rapid and specific LAMP assay for detection and differentiation of the tdh, trh1, and trh2 genes of V. parahaemolyticus and related Vibrio species.
Figures



References
-
- Baba, K., H. Shirai, A. Terai, Y. Takeda, and M. Nishibuchi. 1991. Analysis of the tdh gene cloned from a tdh gene- and trh gene-positive strain of Vibrio parahaemolyticus. Microbiol. Immunol. 35:253-258. - PubMed
-
- Bej, A. K., D. P. Patterson, C. W. Brasher, M. C. L. Vickery, D. D. Jones, and C. A. Kaysner. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tl, tdh and trh. J. Microbiol. Methods 36:215-225. - PubMed
-
- Hill, J., S. Beriwal, I. Chandra, V. K. Paul, A. Kapil, T. Singh, R. M. Wadowsky, V. Singh, A. Goyal, T. Jahnukainen, J. R. Johnson, P. I. Tarr, and A. Vats. 2008. Loop-mediated isothermal amplification assay for rapid detection of common strains of Escherichia coli. J. Clin. Microbiol. 46:2800-2804. - PMC - PubMed
-
- Honda, S., I. Goto, I. Minematsu, N. Ikeda, N. Asano, M. Ishibashi, Y. Kinoshita, N. Nishibuchi, T. Honda, and T. Miwatani. 1987. Gastroenteritis due to Kanagawa negative Vibrio parahaemolyticus. Lancet i:331-332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

