MMP20 hemopexin domain mutation in amelogenesis imperfecta
- PMID: 19966041
- PMCID: PMC3318044
- DOI: 10.1177/0022034509352844
MMP20 hemopexin domain mutation in amelogenesis imperfecta
Abstract
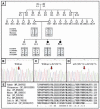
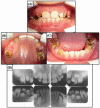
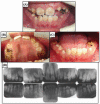
Proteolytic enzymes serve important functions during dental enamel formation, and mutations in the kallikrein 4 (KLK4) and enamelysin (MMP20) genes cause autosomal-recessive amelogenesis imperfecta (ARAI). So far, only 1 KLK4 and 3 MMP20 mutations have been reported in ARAI kindreds. To determine whether ARAI in a family with a hypomaturation-type enamel defect is caused by mutations in the genes encoding enamel proteolytic enzymes, we performed mutational analysis on candidate genes. Mutational and haplotype analyses revealed an ARAI-causing point mutation (c.910G>A, p.A304T) in exon 6 of MMP20 that results in a single amino acid substitution in the hemopexin domain. Western blot analysis showed decreased expression of the mutant protein, but zymogram analysis demonstrated that this mutant was a functional protein. The proband and an affected brother were homozygous for the mutation, and both unaffected parents were carriers. The enamel of newly erupted teeth had normal thickness, but was chalky white and became darker with age.
Figures

References
-
- Bartlett JD, Beniash E, Lee DH, Smith CE. (2004). Decreased mineral content in MMP-20 null mouse enamel is prominent during the maturation stage. J Dent Res 83:909-913 - PubMed
-
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. (2002). Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem 277:49598-49604 - PubMed
-
- Fukae M, Tanabe T, Uchida T, Lee SK, Ryu OH, Murakami C, et al. (1998). Enamelysin (matrix metalloproteinase-20): localization in the developing tooth and effects of pH and calcium on amelogenin hydrolysis. J Dent Res 77:1580-1588 - PubMed
-
- Gomis-Ruth F. (2004). Hemopexin domains. In: Handbook of metalloproteins. Vol. 3 Messerschmidt A, Bode W, Cygler M, editors. Chichester: John Wiley & Sons, Ltd, pp. 631-646
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

