Upregulation of tissue factor in monocytes by cleaved high molecular weight kininogen is dependent on TNF-alpha and IL-1beta
- PMID: 19966052
- PMCID: PMC2822566
- DOI: 10.1152/ajpheart.00825.2009
Upregulation of tissue factor in monocytes by cleaved high molecular weight kininogen is dependent on TNF-alpha and IL-1beta
Abstract
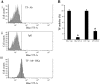
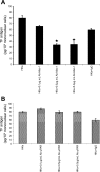
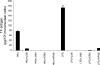
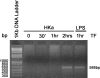
Inflammatory bowel disease and arthritis are associated with contact activation that results in cleavage of kininogen to form high molecular weight kininogen (HKa) and bradykinin. We have previously demonstrated that HKa can stimulate inflammatory cytokine and chemokine secretion from human monocytes. We now show that HKa can upregulate tissue factor antigen and procoagulant activity on human monocytes as a function of time (1-4 h) and HKa concentration (75-900 nM). The amino acid sequence responsible to block HKa effects is G440-H455. The HKa receptor macrophage-1 (Mac-1; CD11b18) is the binding site as shown by inhibition by a monoclonal antibody to CD11b/18. Chemical inhibitors of JNK, ERK, and p38 signaling pathways block cell signaling, as does an inhibitor to the transcription factor NF-kappaB. A combination of monoclonal antibodies to TNF-alpha and IL-1beta but neither alone inhibited the HKa induction of tissue factor. These results suggest that HKa mimics LPS by triggering a paracrine pathway in monocytes that depends on TNF-alpha and IL-1beta. Antibodies to kininogen or peptidomimetics might be a useful and safe therapy in inflammatory diseases or sepsis involving cytokines.
Figures
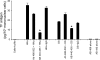
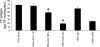
Similar articles
-
High-molecular-weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1, and gC1qR in monocytes.Arterioscler Thromb Vasc Biol. 2006 Oct;26(10):2260-6. doi: 10.1161/01.ATV.0000240290.70852.c0. Epub 2006 Aug 10. Arterioscler Thromb Vasc Biol. 2006. PMID: 16902163 Free PMC article.
-
Regulation of leukocyte recruitment by polypeptides derived from high molecular weight kininogen.FASEB J. 2001 Nov;15(13):2365-76. doi: 10.1096/fj.01-0201com. FASEB J. 2001. PMID: 11689462
-
Nucleolin Mediates LPS-induced Expression of Inflammatory Mediators and Activation of Signaling Pathways.Curr Med Sci. 2020 Aug;40(4):646-653. doi: 10.1007/s11596-020-2229-6. Epub 2020 Aug 29. Curr Med Sci. 2020. PMID: 32862374
-
Inhibition of angiogenesis by cleaved high molecular weight kininogen (HKa) and HKa domain 5.Curr Cancer Drug Targets. 2005 Nov;5(7):519-28. doi: 10.2174/156800905774574039. Curr Cancer Drug Targets. 2005. PMID: 16305348 Review.
-
Inhibition of angiogenesis by a monoclonal antibody to kininogen as well as by kininostatin which block proangiogenic high molecular weight kininogen.Int Immunopharmacol. 2002 Dec;2(13-14):1887-94. doi: 10.1016/s1567-5769(02)00173-x. Int Immunopharmacol. 2002. PMID: 12489802 Review.
Cited by
-
The Many Faces of Cytokine Release Syndrome-Related Coagulopathy.Clin Hematol Int. 2021 Jan 28;3(1):3-12. doi: 10.2991/chi.k.210117.001. eCollection 2021 Mar. Clin Hematol Int. 2021. PMID: 34595461 Free PMC article. Review.
-
Substance P-neurokinin-1 receptor interaction upregulates monocyte tissue factor.J Neuroimmunol. 2012 Jan 18;242(1-2):1-8. doi: 10.1016/j.jneuroim.2011.10.012. Epub 2011 Nov 23. J Neuroimmunol. 2012. PMID: 22115773 Free PMC article.
-
Longitudinal study of circulating protein biomarkers in inflammatory bowel disease.J Proteomics. 2015 Jan 1;112:166-79. doi: 10.1016/j.jprot.2014.09.002. Epub 2014 Sep 16. J Proteomics. 2015. PMID: 25230104 Free PMC article.
-
Automatic gene prioritization in support of the inflammatory contribution to Alzheimer's disease.AMIA Jt Summits Transl Sci Proc. 2014 Apr 7;2014:42-7. eCollection 2014. AMIA Jt Summits Transl Sci Proc. 2014. PMID: 25717399 Free PMC article.
-
A Novel Prothrombotic Pathway in Systemic Sclerosis Patients: Possible Role of Bisphosphonate-Activated γδ T Cells.Front Immunol. 2014 Sep 8;5:414. doi: 10.3389/fimmu.2014.00414. eCollection 2014. Front Immunol. 2014. PMID: 25250025 Free PMC article.
References
-
- Bradford HN, Dela Cadena RA, Kunapuli SP, Dong JF, Lopez JA, Colman RW. Human kininogens regulate thrombin binding to platelets through the glycoprotein Ib-IX-V complex. Blood 90: 1508–1515, 1997 - PubMed
-
- Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA. Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 95: 543–550, 2000 - PubMed
-
- Colman RW, White JV, Scovell S, Stadnicki A, Sartor RB. Kininogens are antithrombotic proteins in vivo. Arterioscler Thromb Vasc Biol 19: 2245–2250, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

