Ins2 deficiency augments spontaneous HLA-A*0201-restricted T cell responses to insulin
- PMID: 19966211
- PMCID: PMC2858386
- DOI: 10.4049/jimmunol.0903414
Ins2 deficiency augments spontaneous HLA-A*0201-restricted T cell responses to insulin
Abstract
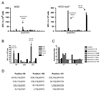
Type 1 diabetes results from the autoimmune destruction of insulin-producing beta cells by T cells specific for beta cell Ags, including insulin. In humans, the non-MHC locus conferring the strongest disease susceptibility is the insulin gene, and alleles yielding lower thymic insulin expression are predisposing. We sought to incorporate this characteristic into an HLA-transgenic model of the disease and to determine the influence of reduced thymic insulin expression on CD8+ T cell responses to preproinsulin. We examined NOD.Ins2(-/-) mice, which do not express insulin in the thymus and show accelerated disease, to determine whether they exhibit quantitative or qualitative differences in CD8+ T cell responses to preproinsulin. We also generated NOD.Ins2(-/-) mice expressing type 1 diabetes-associated HLA-A*0201 (designated NOD.beta2m(-/-).HHD.Ins2(-/-)) in an effort to obtain an improved humanized disease model. We found that CD8+ T cell reactivity to certain insulin peptides was more readily detected in NOD.Ins2(-/-) mice than in NOD mice. Furthermore, the proportion of insulin-reactive CD8+ T cells infiltrating the islets of NOD.Ins2(-/-) mice was increased. NOD.beta2m(-/-).HHD.Ins2(-/-) mice exhibited rapid onset of disease and had an increased proportion of HLA-A*0201-restricted insulin-reactive T cells, including those targeting the clinically relevant epitope Ins B10-18. Our results suggest that insulin alleles that predispose to type 1 diabetes in humans do so, at least in part, by facilitating CD8+ T cell responses to the protein. We propose the NOD.beta2m(-/-).HHD.Ins2(-/-) strain as an improved humanized disease model, in particular for studies seeking to develop therapeutic strategies targeting insulin-specific T cells.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




References
-
- Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235–249. - PubMed
-
- Lucassen AM, Julier C, Beressi JP, Boitard C, Froguel P, Lathrop M, Bell JI. Susceptibility to insulin dependent diabetes mellitus maps to a 4.1 kb segment of DNA spanning the insulin gene and associated VNTR. Nat. Genet. 1993;4:305–310. - PubMed
-
- Bennett ST, Wilson AJ, Cucca F, Nerup J, Pociot F, McKinney PA, Barnett AH, Bain SC, Todd JA. IDDM2-VNTR-encoded susceptibility to type 1 diabetes: dominant protection and parental transmission of alleles of the insulin gene-linked minisatellite locus. J. Autoimmun. 1996;9:415–421. - PubMed
-
- Perez De Nanclares G, Bilbao JR, Calvo B, Vitoria JC, Vazquez F, Castano L. 5'-Insulin gene VNTR polymorphism is specific for type 1 diabetes: no association with celiac or Addison's disease. Ann. N. Y. Acad. Sci. 2003;1005:319–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

