A protein secretion system linked to bacteroidete gliding motility and pathogenesis
- PMID: 19966289
- PMCID: PMC2806738
- DOI: 10.1073/pnas.0912010107
A protein secretion system linked to bacteroidete gliding motility and pathogenesis
Abstract
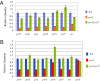
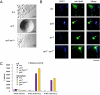
Porphyromonas gingivalis secretes strong proteases called gingipains that are implicated in periodontal pathogenesis. Protein secretion systems common to other Gram-negative bacteria are lacking in P. gingivalis, but several proteins, including PorT, have been linked to gingipain secretion. Comparative genome analysis and genetic experiments revealed 11 additional proteins involved in gingipain secretion. Six of these (PorK, PorL, PorM, PorN, PorW, and Sov) were similar in sequence to Flavobacterium johnsoniae gliding motility proteins, and two others (PorX and PorY) were putative two-component system regulatory proteins. Real-time RT-PCR analysis revealed that porK, porL, porM, porN, porP, porT, and sov were down-regulated in P. gingivalis porX and porY mutants. Disruption of the F. johnsoniae porT ortholog resulted in defects in motility, chitinase secretion, and translocation of a gliding motility protein, SprB adhesin, to the cell surface, providing a link between a unique protein translocation system and a motility apparatus in members of the Bacteroidetes phylum.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Papapanou PN. Epidemiology of periodontal diseases: An update. J Int Acad Periodontol. 1999;1:110–116. - PubMed
-
- Irfan UM, Dawson DV, Bissada NF. Epidemiology of periodontal disease: A review and clinical perspectives. J Int Acad Periodontol. 2001;3:14–21. - PubMed
-
- Armitage GC. Periodontal diseases: Diagnosis. Ann Periodontol. 1996;1:37–215. - PubMed
-
- Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol 2000. 1997;14:216–248. - PubMed
-
- Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

