The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption
- PMID: 19966304
- PMCID: PMC2806780
- DOI: 10.1073/pnas.0912658106
The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption
Abstract
Fertilization triggers a rise in intracellular Ca(2+) concentration ([Ca(2+)](i)) in the egg that initiates a series of events known as egg activation. These events include cortical granule exocytosis that establishes a block to polyspermy, resumption of meiosis, and recruitment of maternal mRNAs into polysomes for translation. Several calcium-dependent proteins, including calcium/calmodulin-dependent protein kinase II (CaMKII), have been implicated in egg activation. However, the precise role of CaMKII in mediating specific events of egg activation and the identity of the isoform(s) present in mouse eggs have not been unequivocally established. Through targeted deletion of the gamma isoform of CaMKII, we find that CaMKIIgamma is the predominant CaMKII isoform in mouse eggs and that it is essential for egg activation. Although CaMKIIgamma(-/-) eggs exhibit a normal pattern of Ca(2+) oscillations after insemination and undergo cortical granule exocytosis, they fail to resume meiosis or to recruit maternal mRNAs. Surprisingly, we find that the recruitment of maternal mRNAs does not directly depend on CaMKII, but requires elevated [Ca(2+)](i) and metaphase II exit. We conclude that CaMKIIgamma specifically controls mouse egg activation by regulating cell cycle resumption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
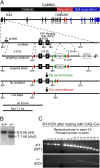
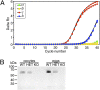
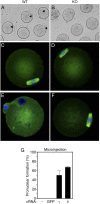
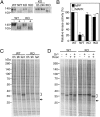
References
-
- Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. - PubMed
-
- Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. - PubMed
-
- Swann K, Saunders CM, Rogers NT, Lai FA. PLCzeta(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol. 2006;17:264–273. - PubMed
-
- Cuthbertson KS, Whittingham DG, Cobbold PH. Free Ca2+ increases in exponential phases during mouse oocyte activation. Nature. 1981;294:754–757. - PubMed
-
- Miyazaki S, Igusa Y. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature. 1981;290:702–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

