Role of the RNA polymerase trigger loop in catalysis and pausing
- PMID: 19966797
- PMCID: PMC2904963
- DOI: 10.1038/nsmb.1732
Role of the RNA polymerase trigger loop in catalysis and pausing
Abstract
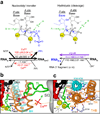
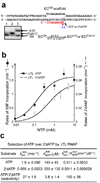
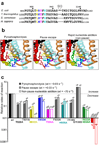
The trigger loop (TL) is a polymorphous component of RNA polymerase (RNAP) that makes direct substrate contacts and promotes nucleotide addition when folded into an alpha-helical hairpin (trigger helices, TH). However, the roles of the TL/TH in transcript cleavage, catalysis, substrate selectivity and pausing remain ill defined. Based on in vitro assays of Escherichia coli RNAP bearing specific TL/TH alterations, we report that neither intrinsic nor regulator-assisted transcript cleavage of backtracked RNA requires formation of the TH. We find that the principal contribution of TH formation to rapid nucleotidyl transfer is steric alignment of the reactants rather than acid-base catalysis, and that the TL/TH cannot be the sole contributor to substrate selectivity. The similar effects of TL/TH substitutions on pausing and nucleotide addition provide additional support for the view that TH formation is rate-limiting for escape from nonbacktracked pauses.
Figures



References
-
- Iyer LM, Koonin EV, Aravind L. Evolution of bacterial RNA polymerase: implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene. 2004;335:73–88. - PubMed
-
- Landick R. Active-site dynamics in RNA polymerases. Cell. 2004;116:351–353. - PubMed
-
- Rhodes G, Chamberlin MJ. Ribonucleic acid chain elongation by Escherichia coli ribonucleic acid polymerase: Isolation of ternary complexes and the kinetics of elongation. J. Biol. Chem. 1974;249:6675–6683. - PubMed
-
- Richardson JP. Loading Rho to terminate transcription. Cell. 2003;114:157–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

