A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome
- PMID: 19966810
- PMCID: PMC2803774
- DOI: 10.1038/nm.2063
A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome
Abstract
The identification of the genes associated with chromosomal translocation breakpoints has fundamentally changed understanding of the molecular basis of hematological malignancies. By contrast, the study of chromosomal deletions has been hampered by the large number of genes deleted and the complexity of their analysis. We report the generation of a mouse model for human 5q- syndrome using large-scale chromosomal engineering. Haploinsufficiency of the Cd74-Nid67 interval (containing Rps14, encoding the ribosomal protein S14) caused macrocytic anemia, prominent erythroid dysplasia and monolobulated megakaryocytes in the bone marrow. These effects were associated with defective bone marrow progenitor development, the appearance of bone marrow cells expressing high amounts of the tumor suppressor p53 and increased bone marrow cell apoptosis. Notably, intercrossing with p53-deficient mice completely rescued the progenitor cell defect, restoring common myeloid progenitor and megakaryocytic-erythroid progenitor, granulocyte-monocyte progenitor and hematopoietic stem cell bone marrow populations. This mouse model suggests that a p53-dependent mechanism underlies the pathophysiology of the 5q- syndrome.
Figures

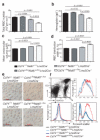
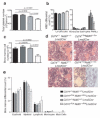
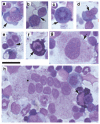
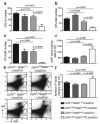
Comment in
-
Myelodysplasia: battle in the bone marrow.Nat Med. 2010 Jan;16(1):30-2. doi: 10.1038/nm0110-30. Nat Med. 2010. PMID: 20057420 No abstract available.
References
-
- Van den Berghe H, et al. Distinct hematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251:437–438. - PubMed
-
- Giagounidis AA, Germing U, Wainscoat JS, Boultwood J, Aul C. The 5q- syndrome. Hematology. 2004;9:271–277. - PubMed
-
- List A, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. - PubMed
-
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. - PubMed
-
- Boultwood J, Lewis S, Wainscoat JS. The 5q-syndrome. Blood. 1994;84:3253–3260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

