The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer
- PMID: 19966862
- PMCID: PMC2837099
- DOI: 10.1038/onc.2009.440
The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer
Abstract
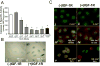
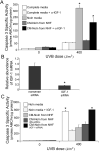
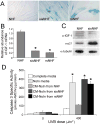
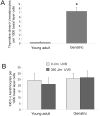
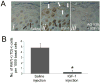
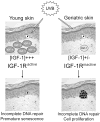
The appropriate response of human keratinocytes to ultraviolet-B (UVB) is dependent on the activation status of the insulin-like growth factor 1 (IGF-1) receptor. Keratinocytes grown in conditions in which the IGF-1 receptor is inactive inappropriately replicate in the presence of UVB-induced DNA damage. In human skin, epidermal keratinocytes do not express IGF-1, and hence the IGF-1 receptor on keratinocytes is activated by IGF-1 secreted from dermal fibroblasts. We now show that the IGF-1 produced by human fibroblasts is essential for the appropriate UVB response of keratinocytes. Furthermore, the expression of IGF-1 is silenced in senescent fibroblasts in vitro. Using quantitative reverse transcriptase-PCR and immunohistochemisty, we can show that IGF-1 expression is also silenced in geriatric dermis in vivo. The diminished IGF-1 expression in geriatric skin correlates with an inappropriate UVB response in geriatric volunteers. Finally, the appropriate UVB response is restored in geriatric skin in vivo through pretreatment with exogenous IGF-1. These studies provide further evidence for a role of the IGF-1 receptor (IGF-1R) in suppressing UVB-induced carcinogenesis, suggest that fibroblasts have a critical role in maintaining appropriate activation of the keratinocyte IGF-1R, and imply that reduced expression of IGF-1 in geriatric skin could be an important component in the development of aging-related non-melanoma skin cancer.
Figures


References
-
- Chuang T-Y, Lewis DA, Spandau DF. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Br J Dermatol. 2005;153:552–557. - PubMed
-
- Cotton J, Spandau DF. Ultraviolet B dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat Res. 1997;147:148–155. - PubMed
-
- Dziadziuszko R, Camidge DR, Hirsch FR. The insulin-like growth factor in lung cancer. J Thoracic Oncol. 2008;3:815–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

