Effects of somatostatin on the responses of rostrally projecting spinal dorsal horn neurons to noxious stimuli in cats
- PMID: 19967064
- PMCID: PMC2788644
- DOI: 10.4196/kjpp.2008.12.5.253
Effects of somatostatin on the responses of rostrally projecting spinal dorsal horn neurons to noxious stimuli in cats
Abstract
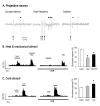
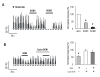
Somatostatin (SOM) is a widely distributed peptide in the central nervous system and exerts a variety of hormonal and neural actions. Although SOM is assumed to play an important role in spinal nociceptive processing, its exact function remains unclear. In fact, earlier pharmacological studies have provided results that support either a facilitatory or inhibitory role for SOM in nociception. In the current study, the effects of SOM were investigated using anesthetized cats. Specifically, the responses of rostrally projecting spinal dorsal horn neurons (RPSDH neurons) to different kinds of noxious stimuli (i.e., heat, mechanical and cold stimuli) and to the Adelta-and C-fiber activation of the sciatic nerve were studied. Iontophoretically applied SOM suppressed the responses of RPSDH neurons to noxious heat and mechanical stimuli as well as to C-fiber activation. Conversely, it enhanced these responses to noxious cold stimulus and Adelta-fiber activation. In addition, SOM suppressed glutamate-evoked activities of RPSDH neurons. The effects of SOM were blocked by the SOM receptor antagonist cyclo-SOM. These findings suggest that SOM has a dual effect on the activities of RPSDH neurons; that is, facilitation and inhibition, depending on the modality of pain signaled through them and its action site.
Keywords: Aδ-fiber; C-fiber; Dorsal horn; Nociception; Pain; Somatostain; Spinal cord.
Figures

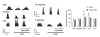
Similar articles
-
Spinal somatostatin superfusion in vivo affects activity of cat nociceptive dorsal horn neurons: comparison with spinal morphine.Neuroscience. 1990;34(3):565-76. doi: 10.1016/0306-4522(90)90165-z. Neuroscience. 1990. PMID: 1972267
-
Presynaptic Inhibition of Primary Nociceptive Signals to Dorsal Horn Lamina I Neurons by Dopamine.J Neurosci. 2018 Oct 10;38(41):8809-8821. doi: 10.1523/JNEUROSCI.0323-18.2018. Epub 2018 Aug 24. J Neurosci. 2018. PMID: 30143577 Free PMC article.
-
Enhanced responses of spinal dorsal horn neurons to heat and cold stimuli following mild freeze injury to the skin.J Neurophysiol. 2001 Aug;86(2):986-96. doi: 10.1152/jn.2001.86.2.986. J Neurophysiol. 2001. PMID: 11495966
-
[Effects of morphine on the activity of various dorsal horn neurons of the spinal cord involved in nociception].Encephale. 1979;5(3):205-13. Encephale. 1979. PMID: 226344 Review. French.
-
PKCγ interneurons, a gateway to pathological pain in the dorsal horn.J Neural Transm (Vienna). 2020 Apr;127(4):527-540. doi: 10.1007/s00702-020-02162-6. Epub 2020 Feb 27. J Neural Transm (Vienna). 2020. PMID: 32108249 Review.
Cited by
-
Somatostatin 2 Receptors in the Spinal Cord Tonically Restrain Thermogenic, Cardiac and Other Sympathetic Outflows.Front Neurosci. 2019 Feb 20;13:121. doi: 10.3389/fnins.2019.00121. eCollection 2019. Front Neurosci. 2019. PMID: 30842723 Free PMC article.
-
Subclinical lipopolysaccharide from Salmonella Enteritidis induces neuropeptide dysregulation in the spinal cord and the dorsal root ganglia.BMC Neurosci. 2019 Apr 25;20(1):18. doi: 10.1186/s12868-019-0502-z. BMC Neurosci. 2019. PMID: 31023212 Free PMC article.
References
-
- Beitz AJ, Shepard RD, Wells WE. The periaqueductal gray- raphe magnus projection contains somatostatin, neurotensin and serotonin but not cholecystokinin. Brain Res. 1983;261:132–137. - PubMed
-
- Chapman V, Dickenson AH. The effects of sandostatin and somatostatin on nociceptive transmission in the dorsal horn of the rat spinal cord. Neuropeptides. 1992;23:147–152. - PubMed
-
- Chrubasik J, Meynadier J, Scherpereel P, Wünsch E. The effect of epidural somatostatin on postoperative pain. Anesth Analg. 1985;64:1085–1088. - PubMed
-
- Dichter MA, Wang HL, Reisine T. Electrophysiological effects of somatostatin-14 and somatostatin-28 on mammalian central nervous system neurons. Metabolism. 1990;39:86–90. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

