Modeling of arrhythmogenic automaticity induced by stretch in rat atrial myocytes
- PMID: 19967066
- PMCID: PMC2788646
- DOI: 10.4196/kjpp.2008.12.5.267
Modeling of arrhythmogenic automaticity induced by stretch in rat atrial myocytes
Abstract
Since first discovered in chick skeletal muscles, stretch-activated channels (SACs) have been proposed as a probable mechano-transducer of the mechanical stimulus at the cellular level. Channel properties have been studied in both the single-channel and the whole-cell level. There is growing evidence to indicate that major stretch-induced changes in electrical activity are mediated by activation of these channels. We aimed to investigate the mechanism of stretch-induced automaticity by exploiting a recent mathematical model of rat atrial myocytes which had been established to reproduce cellular activities such as the action potential, Ca(2+) transients, and contractile force. The incorporation of SACs into the mathematical model, based on experimental results, successfully reproduced the repetitive firing of spontaneous action potentials by stretch. The induced automaticity was composed of two phases. The early phase was driven by increased background conductance of voltage-gated Na(+) channel, whereas the later phase was driven by the reverse-mode operation of Na(+)/Ca(2+) exchange current secondary to the accumulation of Na(+) and Ca(2+) through SACs. These results of simulation successfully demonstrate how the SACs can induce automaticity in a single atrial myocyte which may act as a focus to initiate and maintain atrial fibrillation in concert with other arrhythmogenic changes in the heart.
Keywords: Atrial fibrillation; Automaticity; Modeling; Stretch.
Figures
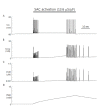
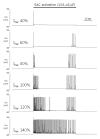
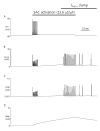
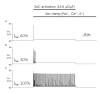
Similar articles
-
Stretch-activated current in human atrial myocytes and Na+ current and mechano-gated channels' current in myofibroblasts alter myocyte mechanical behavior: a computational study.Biomed Eng Online. 2019 Oct 25;18(1):104. doi: 10.1186/s12938-019-0723-5. Biomed Eng Online. 2019. PMID: 31653259 Free PMC article.
-
Role of stretch-activated channels on the stretch-induced changes of rat atrial myocytes.Prog Biophys Mol Biol. 2006 Jan-Apr;90(1-3):186-206. doi: 10.1016/j.pbiomolbio.2005.06.003. Epub 2005 Jul 7. Prog Biophys Mol Biol. 2006. PMID: 16043213 Review.
-
Induced automaticity in isolated rat atrial cells by incorporation of a stretch-activated conductance.Pflugers Arch. 2004 Mar;447(6):819-29. doi: 10.1007/s00424-003-1208-7. Epub 2004 Jan 16. Pflugers Arch. 2004. PMID: 14727114
-
Role of Stretch-activated Channels in the Heart: Action Potential and Ca2+ Transients.2004 Jan 26 [updated 2005 Feb 15]. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. 2004 Jan 26 [updated 2005 Feb 15]. In: Kamkin A, Kiseleva I, editors. Mechanosensitivity in Cells and Tissues. Moscow: Academia; 2005. PMID: 21290758 Free Books & Documents. Review.
-
Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: role of increased troponin C affinity and stretch-activated ion channels.Circ Res. 1998 Nov 30;83(11):1165-77. doi: 10.1161/01.res.83.11.1165. Circ Res. 1998. PMID: 9831710
Cited by
-
Stretch-Activated Current Can Promote or Suppress Cardiac Alternans Depending on Voltage-Calcium Interaction.Biophys J. 2016 Jun 21;110(12):2671-2677. doi: 10.1016/j.bpj.2016.05.026. Biophys J. 2016. PMID: 27332125 Free PMC article.
-
The effects of voltage dependence and ion-binding reaction rates on a thermodynamically constrained mathematical model of the Na/Ca exchanger.Sci Rep. 2025 Jul 1;15(1):21122. doi: 10.1038/s41598-025-07881-y. Sci Rep. 2025. PMID: 40596613 Free PMC article.
References
-
- Baruscotti M, DiFrancesco D, Robinson RB. Na+ current contribution to the diastolic depolarization in newborn rabbit SA node cells. Am J Physiol Heart Circ Physiol. 2000;279:H2303–H2309. - PubMed
-
- Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. - PubMed
-
- Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation. 2000;101:2200–2205. - PubMed
-
- Bode F, Sachs F, Franz MR. Tarantula peptide inhibits atrial fibrillation. Nature. 2001;409:35–36. - PubMed
-
- Boland J, Troquet J. Intracellular action potential changes induced in both ventricles of the rat by an acute right ventricular pressure overload. Cardiovasc Res. 1980;14:735–740. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

