Microecology, intestinal epithelial barrier and necrotizing enterocolitis
- PMID: 19967379
- PMCID: PMC2839249
- DOI: 10.1007/s00383-009-2536-2
Microecology, intestinal epithelial barrier and necrotizing enterocolitis
Abstract
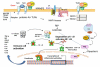
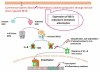
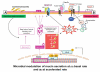
Soon after birth, the neonatal intestine is confronted with a massive antigenic challenge of microbial colonization. Microbial signals are required for maturation of several physiological, anatomical, and biochemical functions of intestinal epithelial barrier (IEB) after birth. Commensal bacteria regulate intestinal innate and adaptive immunity and provide stimuli for ongoing repair and restitution of IEB. Colonization by pathogenic bacteria and/or dysmature response to microbial stimuli can result in flagrant inflammatory response as seen in necrotizing enterocolitis (NEC). Characterized by inflammation and hemorrhagic-ischemic necrosis, NEC is a devastating complication of prematurity. Although there is evidence that both prematurity and presence of bacteria, are proven contributing factors to the pathogenesis of NEC, the molecular mechanisms involved in IEB dysfunction associated with NEC have begun to emerge only recently. The metagenomic advances in the field of intestinal microecology are providing insight into the factors that are required for establishment of commensal bacteria that appear to provide protection against intestinal inflammation and NEC. Perturbations in achieving colonization by commensal bacteria such as premature birth or hospitalization in intensive care nursery can result in dysfunction of IEB and NEC. In this article, microbial modulation of functions of IEB and its relationship with barrier dysfunction and NEC are described.
Figures


References
-
- Sharma R, Young C, Mshvildadze M, et al. Intestinal microbiota: does it play a role in diseases of the neonate? Neoreviews. 2009;10:e166–e179.
-
- Bäckhed F, Ley RE, Sonnenburg JL, et al. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

