Catalytic enantioselective olefin metathesis in natural product synthesis. Chiral metal-based complexes that deliver high enantioselectivity and more
- PMID: 19967680
- PMCID: PMC3517032
- DOI: 10.1002/anie.200904491
Catalytic enantioselective olefin metathesis in natural product synthesis. Chiral metal-based complexes that deliver high enantioselectivity and more
Abstract
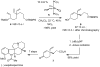
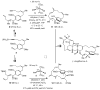
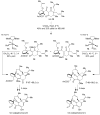
Chiral olefin metathesis catalysts enable chemists to access enantiomerically enriched small molecules with high efficiency; synthesis schemes involving such complexes can be substantially more concise than those that would involve enantiomerically pure substrates and achiral Mo alkylidenes or Ru-based carbenes. The scope of research towards design and development of chiral catalysts is not limited to discovery of complexes that are merely the chiral versions of the related achiral variants. A chiral olefin metathesis catalyst, in addition to furnishing products of high enantiomeric purity, can offer levels of efficiency, product selectivity and/or olefin stereoselectivity that are unavailable through the achiral variants. Such positive attributes of chiral catalysts (whether utilized in racemic or enantiomerically enriched form) should be considered as general, applicable to other classes of transformations.
Figures

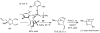

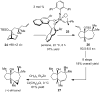



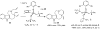
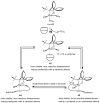



References
-
- Grubbs RH, editor. Handbook of Metathesis. Wiley–VCH; Weinheim: 2003.
-
- Hoveyda AH, Zhugralin AR. Nature. 2007;450:243–251. - PubMed
-
- Houri AF, Xu Z, Cogan DA, Hoveyda AH. J Am Chem Soc. 1995;117:2943–2944.
- Xu Z, Johannes CW, Salman SS, Hoveyda AH. J Am Chem Soc. 1996;118:10926–10927.
- Xu Z, Johannes CW, Houri AF, La DS, Cogan DA, Hofilena GE, Hoveyda AH. J Am Chem Soc. 1997;119:10302–10316.
-
-
For two noteworthy applications to natural product synthesis wherein the reversible nature of catalytic cross-metathesis is utilized, see: Smith AB, III, Adams CM, Kozmin SA, Paone DV. J Am Chem Soc. 2001;123:5925–5937.Fürstner A, Thiel OR, Ackermann L. Org Lett. 2001;3:449–451.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

