Stereoisomerically pure trisubstituted vinylaluminum reagents and their utility in copper-catalyzed enantioselective synthesis of 1,4-dienes containing Z or E alkenes
- PMID: 19967689
- PMCID: PMC3837696
- DOI: 10.1002/anie.200905223
Stereoisomerically pure trisubstituted vinylaluminum reagents and their utility in copper-catalyzed enantioselective synthesis of 1,4-dienes containing Z or E alkenes
Figures

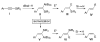
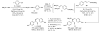
References
-
-
For examples of catalytic enantioselective vinyl additions to carbonyls, see: Oppolzer W, Radinov RN. J. Am. Chem. Soc. 1993;115:1593–1594. Miller KM, Huang W-S, Jamison TF. J. Am. Chem. Soc. 2003;125:3442–3443. Li H, Walsh PJ. J. Am. Chem. Soc. 2004;126:6538–6539. Tomita D, Wada R, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2005;127:4138–4139. Yang Y, Zhu S-F, Zhou C-Y, Zhou Q-L. J. Am. Chem. Soc. 2008;130:14052–14053. Kerrigan MH, Jeon S-J, Chen YK, Carroll PJ, Walsh PJ. J. Am. Chem. Soc. 2009;131:8434–8445. Biradar DB, Gau H-M. Org. Lett. 2009;11:499–502.
-
-
-
For examples of catalytic enantioselective vinyl additions to aldimines, see: Patel SJ, Jamison TF. Angew. Chem. 2004;116:4031–4034. Angew. Chem. Int. Ed. 2004;43:3941–3944. Kong J-R, Cho C-W, Krische MJ. J. Am. Chem. Soc. 2005;127:11269–11276. Ngai M-Y, Barchuk A, Krische MJ. J. Am. Chem. Soc. 2007;129:12644–12645. Lou S, Schaus SE. J. Am. Chem. Soc. 2008;130:6922–6923. Nakao Y, Takeda M, Chen J, Salvi L, Hiyama T, Ichikawa Y, Shintani R, Hayashi T. Chem. Lett. 2008;37:290–291.
-
-
-
For examples of catalytic enantio-selective vinyl conjugate additions to unsaturated carbonyls, see: Oi S, Taira A, Honma Y, Inoue Y. Org. Lett. 2003;5:97–99. Oi S, Sato T, Inoue Y. Tetrahedron Lett. 2004;45:5051–5055. Otomaru Y, Hayashi T. Tetrahedron: Asymmetry. 2004;15:2647–2651. Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew. Chem. 2005;117:3942–3947. Angew. Chem. Int. Ed. 2005;44:3874–3879. Nakao Y, Chen J, Imanaka H, Hiyama T, Ichikawa Y, Duan W-L, Shintani R, Hayashi T. J. Am. Chem. Soc. 2007;129:9137–9143. Vuagnoux-d Augustin M, Alexakis A. Chem. Eur. J. 2007;13:9647–9662. Lee K.-s., Hoveyda AH. J. Org. Chem. 2009;74:4455–4462.
-
-
- Lee Y, Akiyama K, Gillingham DG, Brown MK, Hoveyda AH. J. Am. Chem. Soc. 2008;130:446–447. - PubMed
-
-
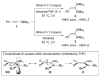
Treatment of aryl-substituted terminal alkynes with dibal-H leads to the generation of significant amounts (≈30%) of the derived alkynylalanes. See: Zweifel G, Snow JT, Whitney CC. J. Am. Chem. Soc. 1968;90:7139–7141.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

