Unlocking fungal cryptic natural products
- PMID: 19967983
- PMCID: PMC3101174
Unlocking fungal cryptic natural products
Abstract
Recent published sequencing of fungal genomes has revealed that these microorganisms have a surprisingly large number of secondary metabolite pathways that can serve as potential sources for new and useful natural products. Most of the secondary metabolites and their biosynthesis pathways are currently unknown, possibly because they are produced in very small amounts and are thus difficult to detect or are produced only under specific conditions. Elucidating these fungal metabolites will require new molecular genetic tools, better understanding of the regulation of secondary metabolism, and state of the art analytical methods. This review describes recent strategies to mine the cryptic natural products and their biosynthetic pathways in fungi.
Figures
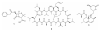
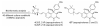
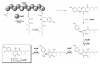
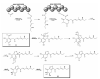
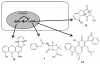
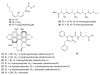

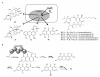
References
-
- Keller NP, Turner G, Bennett JW. Fungal secondary metabolism - from biochemistry to genomics. Nature Review Microbiology. 2005;3:937–947. - PubMed
-
- Schneider P, Misiek M, Hoffmeister D. In vivo and in vitro production options for fungal secondary metabolites. Molecular Pharmaceutics. 2008;5:234–242. - PubMed
-
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Basturkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D’Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Penalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW. Sequencing of Aspergillus nidulans and comparative analysis with. A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. - PubMed
-
- Sanchez JF, Chiang YM, Wang CCC. Diversity of polyketide synthases found in the Aspergillus and Streptomyces genomes. Molecular Pharmaceutics. 2008;5:226–233. - PubMed
-
- Cox RJ. Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Organic & Biomolecular Chemistry. 2007;5:2010–2026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
